Abstract
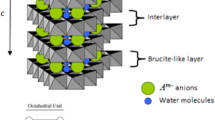
Crystalline aluminum trihydroxides Al(OH)3 (gibbsite, baverite, and nordstrandite) can serve as layered intercalation matrices in which metal salts are arranged in a specific way. Small cations (lithium, magnesium, and transition metals) lie in the octahedral voids of aluminum hydroxide layers, and water molecules are located between the layers. This localization of small cations gives rise to the molecular sieve effect, where alkaline and alkaline earth cations (Na+, K+, Ca2+, etc.), which are large relative to the octahedral voids, are not intercalated into aluminum trihydroxides. In the first step of lithium salt intercalation, the cations, the anions, and the water molecules are incorporated into the interlayer space of aluminum hydroxide with subsequent transition of lithium into the voids of the layer.
Similar content being viewed by others
References
R. Schöllhorn, in:Inclusion Compounds, J. L. Atwood, J. E. Davies, and D. D. MacNicol (eds.), Academic Press, London (1984), pp. 249–334.
D. O’Hare,New J. Chenu.,18, 989–998 (1994).
G. W. Brindley, in:The X-Ray Identification and Crystal Structures of Clay Minerals, Miner. Soc, Clay Minerals Group, London (1961).
E.G. Kukovskii,Structure and Physicochemical Properties of Clay Minerals [in Russian], Naukova Dumka, Kiev (1966).
G. Lagaly,Phil. Trans. R. Soc. Lond.,A311, 315–332 (1984).
J. W. Johnson, A. J. Jacobson, J. F. Brody, and S. M. Rich,Inorg. Chem.,21, 3820–3825 (1982).
J. Votinsky, L. Benes, J. Laousova, and J. Klikorka,Inorg. Chim. Acta,126, 19–23 (1987).
R. Schöllhorn,Angew. Chem. Int. Ed. Engl.,19, 983–1003 (1980).
G. V. Subba Rao and V. F. Shafer,Intercalated Materials, F. A. Levy (ed.), 99–199 (1979).
J. Rouxel,ibid., 201–250.
D.M. McEvan, in:The X-Ray Identification and Crystal Structures of Clay Minerals, Miner. Soc, Clay Minerals Group, London (1961).
K. K. Bissada, W. D. Johns, and F. S. Cheng,Clay Miner.,7, No. 2, 155–166 (1967).
V. C. Farmer and M. M. Mortland,J. Chem. Soc. A, No. 3, 344–351 (1966).
é. V. Sharkina,Structure and Properties of Organomineral Compounds [in Russian], Naukova Dumka, Kiev (1976).
A. Clearfield and R. M. Tindva,J. Inorg. Nucl. Chem.,41, 871 (1979).
Ph. Colomban and Thi M. Pham,Rev. Chim. Min.,22, 143–159 (1985).
Thi M. Pham and Ph. Colomban,Solid State Ion.,17, 295–306 (1986).
H. Kopka, K. Beneke, and G. Lagaly,J. Colloid Interf. Sci.,123, 427–436 (1988).
S. Miyata and T. Kumura,Chem. Lett., No. 8, 843–848 (1973).
G. Mascolo and O. Marino,Miner. Mag.,43, 619–621 (1980).
S. Miyata,Clays Clay Miner.,31, No. 4, 305–311 (1983).
T. Kwon, G. A. Tsigdinos, and T. J. Pinnavaia,J. Am. Chem. Soc,110, 3653–3654 (1988).
M. A. Drezdzon,Inorg. Chem.,27, 4628–4632 (1988).
E. D. Dimotakis and T. J. Pinnavaia,ibid.,29, No. 13, 2393–2394 (1990).
I. Y. Park, K. Kuroda, and C. Kato,Chem. Lett., 2057–2058 (1989).
M. Meyen, K. Beneke, and G. Lagaly,Inorg. Chem.,29, 5201–5207 (1990).
J. Evans, M. Pillinger, and J. Zhang,J. Chem. Soc, Dalton Trans., 2963–2974 (1996).
V. R. L. Constantino and T. J. Pinnavaia,Inorg. Chem.,34, 883–892 (1995).
H. D. Megaw,Zeitschr. Krist.,87, 185–204 (1934).
H. Saalfeld and M. Wedde,ibid.,139, 129–135 (1974).
R. Rothbauer, F. Zigan, and H. O’Daniel,ibid.,125, 317–331 (1967).
F. Zigan, W. Joswig, and N. Burger,ibid.,148, 255–273 (1978).
H. Saalfeld and O. Jarchow,Neues Jahrb. Miner. Abh.,109, 185–191 (1968).
H. J. Bosnians,Acta Crystallogr.,B26, 649–652 (1970).
R. Altaian,Chimia,24, No. 3, 99–108 (1970).
R. Altaian,Acta Crystallogr.,B24, No. 7, 972–977 (1968).
R. Altaian and H. P. Jepsen,Neues Jahrb. Miner. Monat., No. 12, 544–551 (1969).
H. F. W. Taylor,Miner. Mag.,37, No. 287, 338–342 (1969).
H. F. W. Taylor,ibid.,39, No. 304, 377–389 (1973).
G. Brown and M. C. Gastuche,Clay Miner.,7, 193–201 (1967).
R. D. Goodenough, US Patent No. 2964381, Recovery of Lithium,Ref. Zh. Khim., 3K61 (1962).
M. P. Neupert and Ch. K. Bon, US Patent No. 3306700, Method of Lithium Recovery,Ref. Zh. Khim., 10L62P (1969).
J. M. Lee and W. C. Bauman, US Patent No. 4116856, Recovery of Lithium from Brines,Ref. Zh. Khim., 10L41P (1979).
J. M. Lee and W. C. Bauman, US Patent No. 4116858, Recovery of Lithium from Brines,Ref. Zh. Khim., 7L53P (1979).
V. P. Isupov, A. P. Nemudry, N. P. Kotsupalo, and T. I. Samsonova,Chemistry and Technology of Rare Nonferrous Metals and Salts, Abstracts of Papers, Ilim, Frunze (1982), p. 336.
A. P. Nemudry, V. P. Isupov, and N. P. Kotsupalo,Abstracts of Papers from the 6th All-Union Conference on the Chemistry and Technology of Rare Alkaline Elements, Nauka, Moscow (1983), pp. 9–10.
J. L. Burba, US Patent No. 4348295, Crystalline Lithium Aluminates,Ref. Zh. Khim., 12L49P (1983).
A. P. Nemudry, V. P. Isupov, N. P. Kotsupalo, and V. V. Boldyrev,Izv. Sib. Otd. Akad. Nauk SSSR, Ser: Khim. Nauk, No. 11, 28–32 (1984).
A. P. Nemudry, V. P. Isupov, N. P. Kotsupalo, and V. V. Boldyrev,React. Sol.,1, 221–226 (1986).
A. P. Nemudry, V. P. Isupov, N. P. Kotsupalo, and V. V. Boldyrev,Neorg. Khim.,31, No. 5, 651–653 (1986).
A. P. Nemudry, I. A. Poroshina, V. P. Isupov, et al.,Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, No. 2, 48–52 (1987).
V. P. Isupov, “Physicochemical principles underlying methods for processing lithium concentrates isolated from natural brine,” Chemical Sciences Candidate’s Dissertation, Institute of Solid State Chemistry and Mineral Raw Materials, Novosibirsk (1987).
A. P. Nemudry, “Intercalation of lithium salts into hydrargillite,” Chemical Sciences Candidate’s Dissertation, Institute of Solid State Chemistry and Mineral Raw Materials, Novosibirsk (1987).
V. P. Isupov and L. E. Chupakhina,Khim. Ust. Razv.,2, Nos. 2/3, 535–539 (1994).
V. P. Isupov,Zh. Prikl. Khim.,69, No. 1, 12–15 (1996).
V. P. Isupov and L. é Chupakhina, USSR Patent No. 1648900, “Method for preparation of lithium hydroxoaluminates,”Byull. Izobr., No. 18 (1991).
V. P. Isupov, L. é. Chupakhina, N. P. Kotsupalo, and V. V. Boldyrev,Dokl. Akad. Nauk SSSR,316, No. 5, 1144–1146(1991).
V. P. Isupov, A. P. Nemudry, L. é. Chupakhina, and N. P. Kotsupalo,A bstracts of papers from the 9th All-Union Conference on Kinetics and Mechanisms of Chemical Reactions in Solids, Chernogolovka (1986), pp. 174–175.
V. P. Isupov, L. é. Chupakhina, N. P. Kotsupalo, et al.,Dokl. Ross. Akad. Nauk,348, No. 5, 628–630 (1996).
A. P. Nemudry, A. P. Chupakhin, V. P. Isupov, A. Yu. Yagodin, and N. P. Kotsupalo, USSR Patent No. 1289035, “Method for the preparation of lithium hydroxoaluminates of the general formula LiX-2A1(OH)3-nH2O, where X = Cl-, Br-, I-,” No. 3828717 of 10.29.1984.
A. Ya. Yagodin and A. P. Chupakhin,Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, No. 2, 63–66 (1988).
V. A. Pushnyakova, V. P. Isupov, and N. P. Kotsupalo,Abstracts of papers from the 7th All-Union Conference on the Chemistry and Technology of Rare-Earth Alkaline Elements, Apatity (1988), pp. 73–76.
E. F. Allen and H. F. Rodgers,Am. Chem. J.,24, 304–306 (1900).
O. G. Evteeva, V. A. Leonova, and N. P. Kotsupalo,Chemistry and Technology of Alumina [in Russian], Nauka, Novosibirsk (1971), pp. 353–359.
I. V. Guseva, N. P. Kotsupalo, I. S. Lileev, et al.,Rare Alkaline Elements [in Russian], Novosibirsk (1967), pp. 86–91.
V. P. Danilov, I. N. Lepeshkov, and L. T. Kotova,Zh. Neorg. Khim.,12, No. 1, 184–188 (1967).
E. T. Devyatkina, N. P. Tomilov, and A. S. Berger,ibid.,30, No. 1, 86–92 (1985).
C. J. Serna, J. L. White, and L. Stanley,Clays Clay Min.,25, 384–391 (1977).
V. P. Danilov, I. N. Lepeshkov, and L. T. Kotova,Rare and Alkaline Elements [in Russian], Perm (1969), pp. 65–72.
W. Feitknecht,Helv. Chim. Acta,25, 131 (1942).
E. T. Devyatkina, N. P. Kotsupalo, N. P. Tomilov, and A. S. Berger,Zh. Neorg. Khim.,28, No. 6, 1420–1425 (1983).
C. J. Serna, J. L. Rendon, and J. E. Iglesias,Clays Clay Min.,30, No. 3, 180–184 (1982).
J. P. Thiel, C. K. Chiang, and K. R. Poeppelmeier,Chem. Mater.,5, 297–304 (1993).
A. V. Besserguenev, A. M. Fogg, R. J. Francis, et al.,ibid., No. 9, 241–247 (1997).
V. P. Isupov, S. G. Kozlova, S. P. Gabuda, and L. é. Chupakhina,Dokl. Ross. Akad. Nauk,355, No. 6, 774–776 (1997).
V. P. Isupov, L. E. Chupakhina, S. G. Kozlova, and S. P. Gabuda,Proceedings of the 6th European Conference on Solid State Chemistry, Vol. 2, Zurich (1997), PB83.
V. P. Isupov, S. P. Gabuda, S. G. Kozlova, and L. é. Chupakhina,Zh. Strukt. Khim.,39, No. 3, 448–452 (1998).
V. A. Pushnyakova, V. D. Belykh, V. P. Isupov, et al.,Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, No. 6, 57–61 (1984).
V. P. Isupov, V. V. Antsiferova, and L. N. Senchenko,ibid., No. 3, 48–51 (1989).
A. P. Nemudry, I. A. Poroshina, G. N. Goldenberg, et al.,ibid., No. 2, 58–63 (1988).
A. P. Nemudry, V. P. Isupov, N. P. Kotsupalo, and V. V. Boldyrev,ibid., No. 6, 111–114 (1987).
V. P. Isupov, “Intercalation compounds of aluminum hydroxide,” Doctoral Dissertation, Institute of Solid State Chemistry and Mineral Raw Materials, Siberian Branch, Russian Academy of Sciences, Novosibirsk (1998).
G. M. Gusev, L. G. Shumskaya, and N. M. Lemina,Dokl. Akad. Nauk SSSR, No. 4, 921–924 (1977).
S. M. Paramzin, Yu. D. Pankratiev, E. A. Paukshtis, et al.,Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, No. 11, 33–36(1984).
S. M. Paramzin, O. P. Krivoruchko, B. P. Zolotovskii, et al.,ibid., No. 17, 39–48 (1984).
S. M. Paramzin, Yu. D. Pankratiev, V. M. Turkov, et al.,ibid., No. 5, 47–50 (1988).
L. T. Menzheres, V. P. Isupov, and N. P. Kotsupalo,ibid., No. 3, 53–57.
V. P. Isupov, L. T. Menzheres, M. I. Tatarintseva, et al.,ibid., No. 19, 99–104.
N. P. Kotsupalo, L. T. Menzheres, V. P. Isupov, and V. D. Belykh,Abstracts of papers from the 7th All-Union Conference on the Chemistry and Technology of Rare-Eanh Alkaline Elements, Apatity (1988), pp. 12–13.
S. M. Paramzin, “Effect of mechanochemical activation of Al(III) hydroxides on their reactivity and solid state transformations,” Chemical Sciences Candidate’s Dissertation, Novosibirsk (1989).
A. Weiss, H. O. Becker, H. Orth, et al.,Proceedings of the International Clay Conference, Tokyo, 2, 180–184, Israel University Press, Jerusalem (1970).
Author information
Authors and Affiliations
Additional information
Translated fromZhurnal Strukturnoi Khimii, Vol. 40, No. 5, pp. 832–848, September-October, 1999.
Rights and permissions
About this article
Cite this article
Isupov, V.P. Intercalation compounds of aluminum hydroxide. J Struct Chem 40, 672–685 (1999). https://doi.org/10.1007/BF02903444
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02903444