Abstract
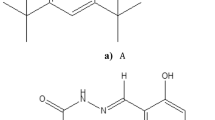
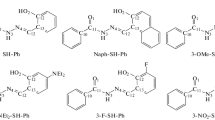
The molecular first hyperpolarizabilities (β) and electronic properties of some azulenic retinal analogues and their derivatives have been investigated theoretically by employing semiempirical approaches. The results indicate that the protonated Schiff bases (PSB) of the 2-substituted azulenic retinal analogues possess extremely large negative β values and very good transparency. These can be attributed to the large difference between the ground state dipole moment and the first excited state dipole moment according to the electronic property analysis. The characteristic blueshifted absorption in polar solvents of the 2-substituted PSB chromophores can be well explained by the negative solvatochromic effects. The largest calculated ¦μβ¦ value can reach the magnitude of 10−44 esu, which is close to the highest reported values of synthesized chromophores.
Similar content being viewed by others
References
Prasad, N. P., Williams, D. J. (eds.), Introduction to Nonlinear Optical Effects in Molecules and Polymers, New York: John Wiley & Sons, 1991.
Fu, G. H., Zhang, C. P., Zhang, G. Y., The optical properties of bacteriorhodopsin and its application to photonics, Physics (in Chinese), 1997, 26: 527.
Clays, K., Hendrickx, E., Triest, M. et al., Nonlinear optical properties of proteins measured by hyper-Rayleigh scattering in solution, Science, 1993, 262: 1419.
Oudar, J. L., Le Person, H., Second-order polarizabilities of some aromatic molecules, Opt. Commum., 1975, 15(2): 258.
Hendrickx, E., Clays, A., Persoons, C. et al., The bacteriorhodopsin chromophore retinal and derivatives: An experimental and theoretical investigation of the second-order optical properties, J. Am. Chem. Soc., 1995, 117: 3547.
Song, Q., Wan, C., Johnson, C. K., Time-resolved second harmonic generation in the randomly oriented purple membrane, J. Phys. Chem., 1994, 98: 1999.
Benjamin, J. C., Molecular materials possessing switchable quadratic nonlinear optical properties, Chem. Eur. J., 1999, 5: 2464.
Asato, A. E., Li, X. Y., Mead, D. et al., Azulenic retinoids and the corresponding bateriorhodopsin analogues: Unusually red-shifted pigments, J. Am. Chem. Soc., 1990, 112: 7398.
Liu, R. S. H., Krogh, E., Li, X. Y. et al., Analyzing the red-shift characteristics of azulenic, naphthyl, other ring-fused and retinyl pigment analogs of bacteriorhodopsin, Photochem. & Photobio., 1993, 58: 701.
Wang, P., Zhu, P. W., Ye, C. et al., Theoretical investigation and molecular design of some azulene derivatives with large hyperpolarizabilities, J. Phys. Chem. A, 1999, 103: 7076.
Asato, A. E., Liu, R. S. H., Rao, V. P. et al., Azulene-containing donor-acceptor compounds as second-order nonlinear chromophores, Tetrahedron Lett., 1996, 37: 419.
Oudar, J. L., Chemla, D. S., Hyperpolarizabilities of the nitroaniline and their relations to the excited state dipole moment, J. Chem. Phys., 1977, 66(5): 2664.
Dalton, L. R., Steier, W. H., Robinson, B. H. et al., From molecules to opto-chips: organic electro-optic materials, J. Mater. Chem., 1999, 9: 1905.
Liu, R. S. H., Muthyala, R. S., Wang, X. S. et al., Correlation of substituent effects and energy levels of the two lowest excited states of the azulenic chromophore, Org. Lett., 2000, 2: 269.
Marder, S. R., Cheng, L. -T., Tiemann, B. G. et al., Large first hyperpolarizabilities in push-pull polyenes by tuning of the bond length alternation and aromaticity, Science, 1994, 263: 511.
Paley, M. S., Harris, J. M., Looser, H. et al., A solvatochromic method for determining second-order polarizabilities of organic molecules, J. Org. Chem., 1989, 54: 3774.
Author information
Authors and Affiliations
About this article
Cite this article
Wang, P., Ye, C. Large negative hyperpolarizabilities (β) of the protonated Schiff bases of the azulenic retinal analogues. Chin.Sci.Bull. 46, 831–835 (2001). https://doi.org/10.1007/BF02900433
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02900433