Summary
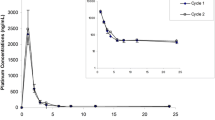
The pharmacokinetics and toxicity of two schedules of etoposide administration were studied in 19 patients suffering from metastatic non-small-cell lung cancer. Ten subjects received a 72-h continuous venous infusion (CVI) of 360 mg/m2 etoposide, and nine were given a daily dose of 120 mg/m2 for 3 consecutive days. In the two groups 80 mg/m2 cis-diamminedichloroplatinum (II) (CDDP) was infused on day 1. With CVI, the steadystate plasma concentration was reached 12–24 h after the start of the treatment. The plasma elimination rate showed a biexponential decay curve in both groups. No significant difference between total body clearance and the β-phase volume of distribution was noted between the two modalities of administration. No relationship was found between biological and pharmacokinetic parameters.
Similar content being viewed by others
References
Aisner J, Echo DA van, Whitacre M, Wiernik P (1982) A phase I trial of continuous infusion VP16-213 (etoposide). Cancer Chemother Pharmacol 7: 157
Arbuck SG, Douglass HO, Crom WR, Goodwin P, Silk Y, Cooper C, Evans WE (1986) Etoposide pharmacokinetics in patients with normal and abnormal organ origin. J Clin Oncol 4: 1690
Batist G, Carney DN, Cowan KH, Veah SR, Gilliom M, Bunn PA, Ihde DC (1986) Etoposide (VP16) and cisplatin in previously treated small-cell lung cancer: clinical trial and in vitro correlates. J Clin Oncol 4: 982
Bennett CL, Sinkule JA, Schilsky RL, Senekjian E, Choi KE (1987) Phase I clinical and pharmacological study of 72-hour continuous infusion of etoposide in patients with advanced cancer. Cancer Res 47: 1952
Brindley CJ, Antoniw P, Newlands ES, Bagshawe KD (1985) Pharmacokinetics and toxicity of the epipodophyllotoxin derivative (VP16-213) in patients with gestational choriocarcinoma and malignant teratoma. Cancer Chemother Pharmacol 15: 66
Canal P, Michel C, Bugat R, Soula G, Carton M (1986) Quantification of teniposide in human serum by high performance liquid chromatography with electrochemical detection. J Chromatogr 375: 451
Clark PI, Slevin ML (1987) The clinical pharmacology of etoposide and teniposide. Clin Pharmacokinet 12: 223
Creagan ET. Richardson RL, Kovach JS (1988) Pilot study of a continuous five-day intravenous infusion of etoposide concomitant with cisplatin in selected patients with advanced cancer. J Clin Oncol 6: 1197
Dhingra HM, Valdivieso M, Booser DJ, Umsaxasdi T, Carr DT, Chuiten DF, Murphy WK, Issel BF, Spitzer G, Farha P, Dixon C (1984) Chemotherapy for advanced adenocarcinoma and squamous cell carcinoma of the lung with etoposide and cisplatin. Cancer Treat Rep 68: 671
D’Incalci M, Rossi C, Zuchetti M, Urso R, Cavalli F, Mangioni C, Willems Y, Sessa C (1986) Pharmacokinetics of etoposide in patients with abnormal renal and hepatic function. Cancer Res 46: 2566
Edwards CM, Glisson BS, King CK, Smallwood-Kentro S, Ross WE (1987) Etoposide-induced DNA cleavage in human leukemia cells. Cancer Chemother Pharmacol 20: 162
Gomeni C, Gomeni R, Rowland M (1978) IG-Pharm: an interactive graphic package for pharmacokinetic analysis. Comput Biomed Res 11: 345
Gouyette A, Deniel A, Pico JL, Droz JP, Baume D, Ostronoff M, Bail N Le, Hayat M (1987) Clinical pharmacology of high-dose etoposide associated with cisplatin. Pharmacokinetic and metabolic studies. Eur J Cancer Clin Oncol 23: 1627
Hsiang YH, Wu HY, Liu LF (1988) Topoisomerases: novel therapeutic targets in cancer chemotherapy. Biochem Pharmacol 37: 1801
Joss RA, Alberto P, Obrecht JP, Barrelet L, Holdener EE, Siegenthaler P, Goldhirsch A, Mermillod B, Cavalli F (1984) Combination chemotherapy for non-small-cell lung cancer with doxorubicin and mitomycin or cisplatin and etoposide. Cancer Treat Rep 68: 1079
Kalminsky DK, Look AT, Ducore J, Fridland A (1983) Effects of the epipodophyllotoxin VP16-213 on cell cycle traverse, DNA synthesis and DNA strand size in cultures of human leukemic lymphoblasts. Cancer Res 43: 1592
Klastersky J (1985) VP16 and cisplatin in the treatment of non-small-cell lung cancer. Semin Oncol 12: 17
Klastersky J (1986) Therapy with cisplatin and etoposide for non-small-cell lung cancer. Semin Oncol 13: 104
Longeval E, Klastersky J (1982) Combination chemotherapy with cisplatin and etoposide in bronchogenic squamous cell carcinoma and adenocarcinoma. Cancer 50: 2751
Matsushima Y, Kanzawa F, Hoshi A, Shimizu E, Nomori H, Sasaki Y, Saijo N (1985) Time-schedule dependency of the inhibiting activity of various anticancer drugs in the clonogenic assay. Cancer Chemother Pharmacol 14: 104
Schabel FM Jr, Trader MW, Laster WR Jr, Corbett TH, Griswold DP Jr (1979)cis-dichlorodiammineplatinum(II): combination chemotherapy and cross-resistance studies with tumors of mice. Cancer Treat Rep 63: 1459
Sinkule JA, Hutson P, Hayes A, Etcubanas E, Evans W (1984) Pharmacokinetics of etoposide (VP16) in children and adolescents with refractory solid tumors. Cancer Res 44: 3109
Slevin ML, Clark PI, Joel SP, Malik S, Osborne RJ, Gregory WM, Lowe DG, Reznek RH, Wrigley PFM (1989) A randomized trial to evaluate the effect of schedule on the activity of etoposide in small cell lung cancer. J Clin Oncol 7: 1333
Sorensen JB, Hansen HH (1988) Combination chemotherapy for advanced adenocarcinoma of the lung. A review. Cancer Chemother Pharmacol 21: 103
Splinter T, Kok T, Kho S, Lameris H, Kate F Ten, Dalesio O, Dolman B, Palmen F, Bouvy J, Burghouts J, Simonis F, Harper P, Rankin E, Reijswoud I van, Hoogenhuijze J van (1988) A multicenter phase II trial of cisplatin and oral etoposide (VP16) in inoperable non-small-cell lung cancer. Semin Oncol 13: 97
Tschopp L, Fliedner VE von, Sauter C, Maurice P, Gratwohl A, Fopp M, Cavalli F (1986) Efficacy and clinical cross-resistance of a new combination therapy (AMSA/VP16) in previously treated patients with acute nonlymphocytic leukemia. J Clin Oncol 4: 318
Author information
Authors and Affiliations
Additional information
This work was supported by a grant from the Comité Départemental de la Haute Garonne de la Ligue Nationale de Lutte Contre le Cancer
Rights and permissions
About this article
Cite this article
Chatelut, E., Chevreau, C., Blancy, E. et al. Pharmacokinetics and toxicity of two modalities of etoposide infusion in metastatic non-small-cell lung carcinoma. Cancer Chemother Pharmacol 26, 365–368 (1990). https://doi.org/10.1007/BF02897295
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02897295