Summary
The effects of various concentrations of Fe3 + (30–125 μM) and various doses of X-irradiation (10 to 30 Gy) on the survival of mouse peritoneal macrophages in culture was tested and compared with the effect of combinations of both Fe3 + and X-irradiation.
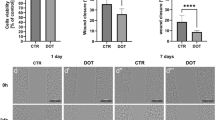
Exposure of the cells to 30 μM Fe3 + or 10 Gy did not significantly alter the survival pattern, as compared with control cells. Greater dosages of iron or X-irradiation caused increasing cellular degeneration and death. When the combined effect of 30 μM Fe3 + and 10 Gy was tested a pronounced decrease in survival time was noted. The production of malondialdehyde (MDA) was studied in cells cultured with and without iron and after exposure to X-irradiation. The results indicated an increasing peroxidative capacity in parallel with increasing dose and extent of exposure to iron and irradiation. Combinations of 30 μM Fe3 + and 10 Gy resulted in enhanced MDA production as compared with these treatments alone (in which levels of MDA did not differ from those in controls).
The findings support the theory that cells containing iron-loaded lysosomes are abnormally sensitive to X-irradiation due to the catalytic activity of the Fe2 +⇌ Fe3 + redox system on lipid peroxidation. The postulated mechanism involves disruption of lysosomal membranes as a consequence of increased lipid peroxidation, resulting in leakage of potent lytic enzymes into the cell sap causing cellular degeneration and death.
Similar content being viewed by others
References
Altaian KI (1970) In: Radiation biochemistry I. New York, Academic Press, p 77–102
Arborgh B, Brunk U, Ericsson JLE (1974) Osmotic pressure and other factors influencing the fine structure and localization of acid phosphatase in glutaraldehyde-fixed cells and tissues. In: Sanders JV and Goodchild DJ (eds) Electron microscopy: Proc 8th Int Congr Electron Microsc, Canberra, Austr. Austr Acad Sci, vol II, p 146
Barber AA (1966) Lipid peroxidation in rat tissue homogenates: interaction of iron and ascorbic acid as the normal catalytic mechanism. Lipids 1:146–151
Baserga R (1968) Biochemistry of the cell cycle: a review. Cell Tissue Kinet 1:167–191
Blozis GG, Robinson JE (1968) Oral tissue changes caused by radiation therapy and their management. Dent Clin N Am 12:643–657
Brunk U, Ericsson JLE (1972) The demonstration of acid phosphatase in in vitro cultured cells. Studies on the significance of fixation, tonicity and permeability. Histochem J 4:349–363
Collins P, Brunk U, Arborgh B (1977) A comparison of the effects of three widely used glutaraldehyde fixatives on cellular volume and structure. A TEM, SEM, volumetric and cytochemical study. Acta Pathol Microbiol Scand, Section A 85: 157–168
Epifanova OI, Terskikh VV (1969) Review Article: On the resting periods in the cell life cycle. Cell Tissue Kinet 2:75–93
Fong KL, McCay PB, Poyer LJ (1973) Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem 248:7792–7797
Goldstein IM, Weissmann G (1977) Intracellular digestion: lysosomes and cellular injury. In: Handbook of physiology. American Physiological Society, Bethesda, pp 603–613
Gross NJ, Balis JV (1978) Functional, biochemical and morphologic changes in alveolar macrophages following thoracic X-irradiation. Lab Invest 39:381–389
Gruger Jr EH, Tappel AL (1969) Reactions of biological antioxidants: 1. Fe3-catalyzed reactions of lipid hydroperoxides with α-tocopherol. Lipids 5:326–331
Halliwell B (1981) The biological effects of the Superoxide radical and its products. Bull Eur Physiopathol Resp 17 (suppl): 21–28
Hamberg H, Brunk U, Ericsson JLE (1976) Cytoplasmic effects of X-irradiation on cultured cells in a non-dividing stage. 1. Establishment of an experimental model. Acta Pathol Microbiol Scand, Section A, 84:201–214
Henkin RI, Lippoldt RE, Bilstad J, Edelhoch H (1975) A Zink protein isolated from human parotid saliva. Proc Natl Acad Sci, USA 72:488–492
Högberg J, Moldeus P, Arborgh B, O’Brien PJ, Orrenius S (1975) The consequences of lipid peroxidation in isolated hepatocytes. Eur J Biochem 59:457–462
Kashima HK, Kirkham WR, Andrews RJ (1965) Postirradiation sialoadenitis. A study of the clinical features, histopathologic changes and serum enzyme variations following irradiation of human salivary glands. Am J Roentgen Rad Ther Nucl Med 94:271–291
Kergonou JF, Bernard P, Braquet M, Rocquet G (1981) Effect of whole-body gamma irradiation on lipid peroxidation in rat tissues. Biochimie 63:555–559
Mead JF (1976) Free radical mechanisms of lipid damage and consequences for cellular membranes. In: Pryor WA (ed) Free radicals in biology. Academic Press, New York, pp 51–69
Mira JG, Wescott WB, Starcke EN, Shannon IL (1981) Some factors influencing salivary function when treating with radiotherapy. Int J Rad Oncol Biol Phys 7:535–541
Nathan C, Cohn Z (1980) Role of oxygen-dependent mechanisms in antibody-induced lysis of tumor cells by activated macrophages. J Exp Med 152:198–208
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Peel DM, Coggle JE (1980) The effect of X-irradiation on alveolar macrophages in mice. Rad Res 81:10–19
Powers EL (1972) The hydrated electron, the hydroxyl radical and hydrogen peroxide in radiation damage in cells. Isr J Chem 10:1199–1211
Poyer LJ, MacCay PB (1971) Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. J Biol Chem 246:263–269
Puck TT, Marcus PI (1956) Action of X-rays on mammalian cells. J Exp Med 103:653–667
René AA, Darden JH, Parker JL (1971) Radiation-induced ultrastructural and biochemical changes in lysosomes. Lab Invest 25:230–239
Ribarov SR, Benov LC (1981) Relationship between the hemolytic action of heavy metals and lipid peroxidation. Biochim Biophys Acta 640:721–726
Shafer WG (1953) The effect of single and fractionated doses of selectively applied X-ray irradiation on the histologic structure of the major salivary glands of the rat. Dent Res 32:796–806
Sinn DP, Stoker NG, Epker BN (1972) Effects of fractionated doses of cobalt 60 irradiation on rabbit submandibular glands: light microscopic studies. J Oral Surg 30:277–283
Spencer H, Samachson J (1967) Secretion of alkaline earth metals in saliva. In: Schneyer LH, Schneyer CA (eds) Secretory mechanism of salivary glands. Academic Press, New York, pp 101–113
Svingen BA, O’Neal FO, Aust SD (1978) The role of Superoxide and singlet oxygen in lipid peroxidation. Photochem Photobiol 28:803–809
Tappel AL (1975) Lipid peroxidation and fluorescent molecular damage to membranes. In: Trump BF, Arstila AU (eds) Pathobiology of cell membranes. Academic Press, New York, pp 145–170
Ueno AM, Goldin EM, Cox AB, Lett JT (1979) Deficient repair and degradation of DNA in X-irradiated L5178YS/S cells: cell-cycle and temperature dependence. Rad Res 79:377–389
Weiss SJ, Lobuglio AF, Kessler HB (1980) Oxidative mechanisms of monocyte-mediated cytotoxicity. Proc Natl Acad Sci USA 77:584–587
Wills ED, Wilkinson AE (1966) Release of enzymes from lysosomes by irradiation and the relation of lipid peroxide formation to enzyme release. Biochem J 99:657–667
Wills ED (1965) Mechanisms of lipid peroxide formation in tissues: role of metals and haematin proteins in the catalysis of the oxidation of unsaturated fatty acids. Biochim Biophys Acta 98:238–251
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abok, K., Blomquist, E., Ericsson, J. et al. Macrophage radiosensitivity in culture as a function of exposure to ionic iron. Virchows Archiv B Cell Pathol 42, 119–129 (1983). https://doi.org/10.1007/BF02890375
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02890375