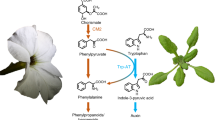
Abstract
The transaminations of L-tryptophan (L-trp) and of L-phenylalanine (L-phe) are catalysedin vitro by the same non-specific aminotransferase. The transaminations procceed at the same pH (pH 8.5) and temperature (45 °C) optima, have parallel increases in activity with addition of the coenzyme pyridoxal phosphate (PRP) and have identical elution characteristics in gel chromatography. The enzyme from pea seedlings has a relatively weak affinity for both amino acids (Km L-trp = 4.16 × 10−1 mmol 1−1; Km L-phe = 2.10 × 10−1 mmol 1−1). Differences in affinity for a series of keto acids in the pea enzyme were observed, with pyruvate having the strongest and glyoxylate the weakest affinity. Transamination of L-trp and L-phe was demonstrated by enzyme extracts from pea, maize and tomato, but was not detected in kohlrabi. The amino acids L-asparagine (L-asn), L-phe, L-lysine (L-lys), L-methionine (L-met) have distinct inhibitory effects on the transamination of L-trp. Indolylacetylaspartate and tryptophol were shown to be competitive inhibitors. The regulation at the molecular level of L-trp transaminase activity is discussed.
Similar content being viewed by others
Abbreviations
- L-trp:
-
L-tryptophan
- L-phe:
-
L-phenylalanine
- L-asp:
-
L-aspartic acid
- L-lys:
-
L-lysine
- L-met:
-
L-methionine
- PRP:
-
pyridoxal phosphate
- IAA:
-
indoly1-3-acetic acid
- IPyA:
-
indolyl-3-pyruvic acid
- IAAsp:
-
indolylacetylaspartic acid
- ILA:
-
indolyl-3-lactic acid
- IAN:
-
indolyl-3-acetonitrile
- T-NH2 :
-
tryptamine
- PPyA:
-
phenylpyruvic acid
- PyA:
-
pyruvic acid
- KG:
-
α-ketoglutarate
- T-OH:
-
tryptophol
- TAT:
-
L-tryptophan aminotransferase
- PAT:
-
L-phenylalanine aminotransferase
References
Bradford, M. M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. - Anal. Biochem.72: 248–254, 1976.
Brown, H. M., Purves, W. K.: Indoleacetaldehyde reductase ofCucumis sativum L. Kinetic properties and role in auxin biosynthesis. - Plant Physiol.65: 107–113, 1980.
Chen, J., Boll, W. G.: Tryptophan synthetase in shoot and root tissue of pea seedlings. - Can. J. Bot.46: 1031–1041, 1968.
El Bahr, M. K., Kutáček, M., Opatrny, Z., Li Sok Nam, Prasad, R.: Changes in L-tryptophan and auxin content during the life cycle of normal and tumorous tobacco cell culture. - Biochem. Physiol. Pflanz.179: 739–748, 1984.
Forest, J. C., Wightman, F.: Amino acid metabolism in plants II. Transamination reaction of free amino acids in cell free extract of cotyledons and growing tissues of bushbean seedlings (Phaseolus vulgaris L.). - Can. J. Biochem.50: 538–542, 1972.
Gamborg, O. L., Wetter, L. R.: An aromatic amino acid transaminase from mung bean. - Can. J. Biochem. Physiol.41: 1733–1740, 1963.
Kutáček, M.: Auxin biosynthesis and its regulation on the molecular level. - Biol. Plant.27: 145–153, 1985.
Kutáček, M., Terziivanova-Dimova, S. D.: Proposed enzymes of auxin synthesis and their regulation III. Some properties of pea indolylacetyldehyde oxidase. - Biol. Plant., in press.
Kutáček, M., Kefeli, V. I.: The present knowledge of indole compounds in plants of theBrassicaceae family. - In: Wightman, F., Setterfield, G. (ed.): Biochemistry and Physiology of Plant Growth Substances, Pp. 127–152. The Runge Press, Ottawa 1968.
Kutáček, M., Procházka, Ž.: Methodes de détermination et d’isolement des composés indoliques chez les Cruciferes. - In: Régulateurs Naturels de la Croissance Végétale. (Colloques de CNRS. Vol. 123.) Pp. 445–456. CNRS, Paris 1964.
Langer, I., Stránský, P., Kutáček, M.: Differences of cytoplasmic transaminase activity in normal and opaque maize (Zea mays L.) seedlings. - Theor. appl. Genet.46: 19–23, 1975.
Lineweaver, H., Burk, D.: The determination of enzyme dissociation constant. - J. amer. chem. Soc.56: 658–666, 1934.
Liu, S. T., Katz, C. D., Knight, C. A.: Indole-3-acetic acid synthesis in tumorous and nontumorous species ofNicotiana. - Plant Physiol.61: 743–747, 1978.
Mahadevan, S., Stowe, B.: Conversion of 3-indole-acetaldoxime to glucobrassicin and sulphoglucobrassicin by woad (Isatis tinctoria L.). - In: Carr, D. (ed.): Plant Growth Substances 1970. Pp. 117–126. Springer Verlag, Heidelberg 1972.
Matheron, M. E., Moore, T. O.: Properties of an aminotransferase of pea (Pisum sativum L.) - Plant Physiol.52: 63–67, 1973.
Moloney, M. M., Elliott, M. C.: Tryptophan and indole-3-acetic acid accumulation inAcer cell cultures and its relationship with cell autolysis. - Planta156: 326–331, 1982.
Noguchi, T., Hayashi, S.: Peroxisomal localization and properties of tryptophan aminotransferase in plant leaves. - J. biol. Chem.255: 2267–2269, 1980.
Truelsen, T. A.: Indole-3.pyruvic acid as an intermediate in the conversion of tryptophan to indole-3-acetic acid. Some characterizations of tryptophan transaminase from mung bean seedlings. - Physiol. Plant.26: 289–295, 1972.
Truelsen, T. A.: Indole-3-pyruvic acid as an intermediate in the conversion of tryptophan to indole-3-acetic acid II. Distribution of tryptophan transaminase activity in plants - Physiol. Plant.28: 67–70, 1973.
Wightman, F., Cohen, D.: Intermediate steps in the enzymatic conversion of tryptophan to IAA in cell. - In: Wightman, F., Setterfield, G. (ed.): Biochemistry and Physiology of Plant Growth Substances. Pp. 273–288. The Runge Press, Ottawa 1968.
Wightman, F., Rauthan, B. S.: Evidence for the biosynthesis and natural occurence of the auxin phenylacetic acid in higher plants. - In: Plant Growth Substances 1973. Pp. 15–27, Hirokawa, Tokyo 1974.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Terziivanova-Dimova, S.D., Kutáček, M. Enzymes of auxin biosynthesis and their regulation I. Tryptophan and phenylalanine aminotransferase in pea plants. Biol Plant 33, 277–286 (1991). https://doi.org/10.1007/BF02885374
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02885374