Abstract
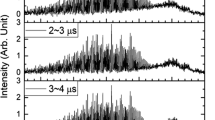
The vibrational energy transfer from highly vibrationally excited CO to H2O molecules is studied by time-resolved Fourier transform infrared emission spectroscopy (TR FTIR). Following the 193 nm laser photolysis of CHBr3 and O2 the secondary reactions generate CO(v). The infrared emission of CO(v → v−1) is detected by TR FTIR. The excitation of H2O molecules is not observed. By the method of the spectral simulation and the differential technique, 8 rate constants for CO(v)/H2O system are obtained: (1.7 ±0.1), (3.4 ±0.2), (6.2 ±0.4), (8.0 ±1.0), (9.0 ±2.0), (12 ±3), (16 ±4) and (18 ±7) (1013cm3 · molecule-1· s-1). At least two reasons lead to the efficient energy transfer. One is the contributions of the rotational energy to the vibational energy defect and the other is the result of the complex collision. With the SSH andab initio calculations, the quenching mechanism of CO(v) by H2O is suggested.
Similar content being viewed by others
References
Stephenson, J. C., Mosburg, Jr. E. R., Vibrational energy transfer in CO from 100 to 300 K,J. Chem. Phys., 1974, 60: 3562.
Buchwald, M. I., Bauer, S. H., Vibrational relaxation in CO2 with selected collision partners, I H2O and D2O,J. Phys. Chem., 1972, 76: 3108.
Kurian, J., Sreekanth, A. K., Laser schlieren study of vibrational relaxation of N2 by H2O,Chem. Phys., 1987, 114: 295.
Shin, H. K., Importance of complex-mode collisions in the vibrational relaxation of strongly attracting molecules, the H2O-HC1 system,Chem. Phys. Lett., 1993, 216(1,2): 27.
Lieb, S. G., Bevan, J. W., A traped collsion pair approach to vibrational predissoeiation in hydrogen-bonded complexes,Chem. Phys. Lett., 1985, 122(3): 284.
Wang Baoshan, Gu Yueshu, He Yonget al., The v-v energy transfer of highly vibrationally excited states (I) —Vibrational quenching of CO(v) by CO2,Chinese Science Bulletin, 1998, 43(17): 1536.
Wang, X. B., Li, H. Z., Kong, F. A.et al., The vibrational quenching of NO(v = 1–11) by N2O studied by time-resolved Fourier transform infrared emission spectroscopy,Chem. Phys. Lett., 1993, 208(3,4): 290.
Bott, J. F., Vibrational relaxation of DF(v = 1–4) in D2,H2, N2,HF and CO2,J. Chem. Phys., 1979, 70(9): 4123.
Schwartz, R. N., Slawsky, Z. I., Herzfeld, K. F., Calculation of vibrational relaxation times in gases,J. Chem. Phys., 1952, 20: 1591.
Yaron, D., Peterson, K. I., Zolandz, D., Water hydrogen bonding: The structure of the water-carbon monoxide complex,J. Chem. Phys., 1990, 92: 7095.
Shin, H. K., Self-relaxation of vibrationally excited H2O molecules,J. Chem. Phys., 1993, 98(3): 1964.
Author information
Authors and Affiliations
About this article
Cite this article
Wang, B., Gu, Y., Li, Q. et al. The v-v energy transfer of highly vibrationally excited states (II). Chin. Sci. Bull. 43, 1621–1625 (1998). https://doi.org/10.1007/BF02883406
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02883406