Abstract
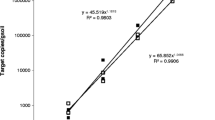
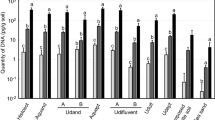
A quantitative PCR assay based on the competitive PCR technique was compared to the classical soil dilution (SD) method for its ability to estimateV. dahliae propagules directly in soils collected from fields under potato production. A strong correlation (r = 0.97) was observed betweenV. dahliae propagules estimated using the quantitative PCR assay and those using the SD method. Coamplification ofV. dahliae DNA with competitor DNA provided accurate quantification in the range of 102 to 107 spores and 1 to 100 microsclerotia/g of soil. The number ofV. dahliae propagules detected in PEI soils ranged from 4.9 to 15.6 and 0.06 to 0.5 microsclerotia/g of soil for PCR assay and SD method, respectively. The strong correlation between PCR assay and SD method and the non significant differences between replications of PCR estimates ofV. dahliae propagules in soils (P< 0.05) show that the PCR assay is reliable and reproducible, and comparable to the SD method. This method is fast, does not depend on the subjectiveness of the traditional plating method, and offers an improvement in speed and precision over currently used methods. In addition, it can be extended to estimateV. dahliae propagules in other pathosystems and finds immediate and practical use in epidemiological studies to determine the effects of various crop management strategies on the dynamics and level of fungal propagules in the soil in order to establish threshold levels for assessing disease risks and develop disease prediction systems.
Resumen
Se comparó un ensayo cuantitativo de PCR basado en la técnica competitiva de PCR con el método clásico de dilucón del suelo (SD), por su capacidad de estimar propágulos de V. dahliae directamente en suelos colectados de otros campos de producción de papa. Se observó una fuerte correlación (r = 0.97) entre los propágulos de V. dahlia estimados usando el análisis cuantitativo de PCR con los usados en el método de dilución (DS). La co-amplificación del ADN de V. dahliae con el ADN competidor proporcionó cuantificación exacta en el rango de 102 a 107 esporas y de 1 a 100 microesclerotias por gramo de suelo. El número de propágulos de V. dahlie dectados en suelos PEI fluctuaron entre 4.9 a 15.6 y entre 0.06 a 0.5 microesclerotia/g de suelo por análisis de PCR y método de DS, respectivamente. La sólida correlación entre el análisis de PCR estimadas de los propágulos de V. dahlie en suelos (P<0.05), muestra que el análisis de PCR es confiable y reproducible y comparable con el método DS. Este método es rápido, no depende de las subjectividades de los métodos tradicionales de siembra y ofrece una mejoría en cuanto a velocidad y precisión respecto de otros métodos actualmente en uso. Además, puede expandirse par estimar los propágulos de V. dahlie en otros patosistemas y encontrar un uso inmediato y práctico en estudios epidemioloógicos par determinar los efectos de diversas estrategias de manejo de cultivos en la dinámica y nivel de propágulos de hongos en el suelo, con el fin de establecer niveles de inicio para evaluar los riesgos de la enfermedad y desarrollar sistemas de predicción de la enfermedad.
Similar content being viewed by others
Literature Cited
Ashworth, L.J., J.E. Waters, A.G. George, and O.D. McCutcheon. 1972. Assessment of microsclerotia ofVerticillium albo-atrum in field sols. Phytopathology 62:715–719.
Butterfield, E.J., and J.E. DeVay. 1977. Reassessment of soil assays forVerticillium dahliae. Phytopathology 67:1073–1078.
Celetti, M.J., and H.W. Platt. 1987. A new cause for an old disease:Verticillium dahliae found on Prince Edward Island. Am Potato J 64:209–212.
DeVay, J.E., L.L. Forrester, R.H. Garber, and E.J. Butterfield. 1974. Characteristics and concentrations of propagules ofVerticillium dahliae in air-dried field soils in relation to the prevalence of verticillium wilt in cotton. Phytopathology 64:22–29.
Harris, D.C., J.R. Yand, and M.S. Ridout. 1993. The detection and estimation ofVerticillium dahliae in naturally infested soil. Plant Pathol 42:238–250.
Harris, H.D., and Yang, Y.R. 1996. The relationship between the amount ofVerticillium dahliae in soil and the incidence of strawberry wilt as a basis for disease risk prediction. Plant Pathol. 45:106–114.
Hawke, M.A., and G. Lazarovits. 1994. Production and manipulation of individual microsclerotia ofVerticillium dahliae for use in studies of survival. Phytopathology 84:883–890.
Heinz, R.A., and H.W. Platt. 2000a Improved DNA extraction method for Verticillium detection and quantification in large-scale studies using PCR-based techniques. Can J Plant Pathol 22:117–121.
Heinz, R.A., and H.W. Platt. 2000b. A compettive PCR-based assay to quantifyVerticillium tricorpus propagules in soil. Can J Plant Pathol 22:122–130.
Hu, X., R.N. Nazar, and J.E. Robb. 1993. Quantification ofVerticillium biomass in wilt disease development. Physiol Mol Plant Pathol 42:23–26.
Huisman, O.C., and L.J. Ashworth. 1974. Quantitative assessment ofVerticillium albo-atrum in field soils: procedural and substrate improvements. Phytopathology 64:1043–1044.
Huisman, O.C. 1988. Seasonal colonization of roots of field-grown cotton byVerticillium dahliae andV. tricorpus. Phytopathology 78:708–716.
Liu, H.W., P.H. Goodwin, and C.R. Kuske. 1994. Quantification of DNA from the aster yellows mycoplasma-like organism in aster leafhoppers (Maerosteles fasifrons Stal) by a competitive polymerase chain reaction. Syst Appl Microbiol 1:75–78
Mahuku, G.S., P.H. Goodwin, and R. Hall. 1995. A competitive polymerase chain reaction to quantify DNA ofLeptosphaeria maculans during blackleg development in oilseed rape. Mol Plant Microbe Int 8:761–767
Mahuku, G.S., H.W. Platt, and P. Maxwell. 1999. Comparison of traditional and polymerase chain reaction-based assays to detect and identify Verticillium wilt pathogens of potato. Can J Plant Pathol 21:125–131.
MacDougall, J.I., C. Veer, and F. Wilson. 1988. Soils of Prince Edward Island: Prince Edward Island Soil Survey, 1988. Agriculture Canada Land Resources Research Institute Contribution No. 83-54. Agriculture Canada, Ottawa, 210 p.
Nicholson, P., D.R. Simpson, G. Weston, H.N. Rezanoor, A.K. Lees, D.W. Parry, and D. Joyce. 1998. Detection and quantification ofFusarium culmorum andFusarium graminearum in cereals using PCR assays. Physiol Mol Plant Pathol 53:17–37.
Platt, H.W. 1986. Varietal response and crop loss due to Verticillium wilt of potato caused byV. albo-atrum. Phytoprotection 67:123–127.
Powelson, M.L., and R.C. Rowe. 1993. Biology and management of early dying of potatoes. Annu Rev Phytopathol 31:111–126.
Robb, J., X. Hu, H.W. Platt, and R.N. Nazar. 1994. PCR-based assays for the detection and quantification of Verticillium species in potato. In: Schots, A., F.M. Dewey and R. Oliver (eds), Modern Assays for Plant Pathogenic Fungi: Identification, Detection and Quantification. CAB International pp. 83–90.
Robb, J., R. Moukhamedov, X. Hu, H.W. Platt, and R.N. Nazar. 1993. Putative subgroups ofVerticillium albo-atrum distinguishable by PCR-based assays. Physiol. Mol. Plant Pathol. 43:423–436.
Rowe, R.C., J.R. Davis, M.L. Powelson, and D.I. Rouse. 1987. Potato early dying: causal agents and management strategies. Plant Dis 71:482–489.
Sturz, A.V., and J.E. Diamond. 1997. Verticillium wilt of potatoes. PEI Canada Fact Sheet AGDEX # 161–92/1, Prince Edward Island, Department of Agriculture and Forestry, Charlottetown, P.E.I. Canada Fact Sheet AGDEX #161–92/1
Termorshuizen, A.J., J.R. Davis, G. Gort, D.C. Harris, O.C. Huisman, G. Lazarovits, T. Locke, J.M. Melero Vara, L. Mol, E.J. Paplomatas, H.W. Platt, M. Powelson, D.I. Rouse, R.C. Rowe, and L. Tsror. 1998. Inter-laboratory comparison of methods to quantify microsclerotia ofVerticillium dahliae in soil. Appl Environ Microbiol 64:3846–3853
Volossiouk, T., J.E Robb, and R.N. Nazar. 1995. Direct extraction of DNA for PCR-mediated assays of soil organisms. Appl Environ Microbiol 61:3972–3976.
Wheeler, T.A., and R.C. Rowe. 1995. Influence of soil characteristics and assay techniques on quantification ofVerticillium dahliae in Ohio soils. Plant Disease 79:29–35.
Zhou, J., M.A. Bruns, and J.M. Tiedje. 1996. DNA recovery from soils of diverse composition. Appl Environ Microbiol 62:316–322.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mahuku, G.S., Platt, H.W. QuantifyingVerticillium dahliae in soils collected from potato fields using a competitive PCR assay. Am. J. Pot Res 79, 107–117 (2002). https://doi.org/10.1007/BF02881519
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02881519