Abstract
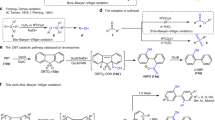
Rose Bengal-sensitized photo-oxygenations of several bisphenylhydrazones of 1,2-diketones have been studied. The photo-oxygenation of benzil bisphenylhydrazone (9a) gave a mixture of products consisting of 2,4,5-triphenyl-1,2,3-triazole (10a, 27%), benzil (11a, 16%) benzoic acid (12, 33%) and biphenyl (13, 6%), whereas, biacetyl bisphenylhydrazone (9b), under analogous conditions gave a 2% yield of 4,5-dimethyl-2-phenyl-1,2,3-triazole (10b), a 30% yield of biacetyl monophenylhydrazone (16b) and a 1% yield of biphenyl. Cyclohexane-1,2-dione bisphenylhydrazone (9c) gave likewise, the corresponding triazole,10c (19%), cyclohexane-1,2-dione monophenylhydrazone (16c, 18%) and a small amount of biphenyl (13, 2%). Photo-oxygenation of acenaphthenequinone bisphenylhydrazone (9d) gave a mixture of products consisting of acenaphthenequinone monophenylhydrazone (16d, 67%), 1,8-naphthoic anhydride (23, 22%) and biphenyl (13, 9%). The photo-oxygenations of both benzil monophenylhydrazone (16a) and acenaphthenequinone monophenylhydrazone (16d) gave rise to products, similar to those obtained from9a and9d, respectively.
Photo-oxygenation of 1,2-bisphenylazostilbene (18a) gave a 65% yield of the triazole10a, whereas, 2,3-bisphenylazo-2-butene (18b) gave a 16% yield of the triazole10b and a 5% yield of biphenyl. On the other hand, the photo-oxygenation of 1,2-bisphenylazocyclohexene (18c) gave a 77% yield of cyclohexane-1,2-dione (11c). The photo-oxygenation of 1,2-bisphenylazoacenaphthylene (18d) gave a 46% yield of acenaphthenequinone monophenylhydrazone (16d), a 51% yield of 1,8-naphthoic anhydride (23) and a 9% yield of biphenyl.
The photo-oxygenations of both benzil bishydrazone (25) and benzil monohydrazone (27) have been studied. In the case of the former, a mixture of products consisting of a 28% yield of benzil (11a) and a 17% yield of benzoic acid have been isolated, whereas in the case of the latter, a 55% yield of benzoic acid was isolated as the sole product.
Reasonable mechanisms have been suggested for the formation of the various products in the photo-oxygenations of the different substrates that we have examined.
Similar content being viewed by others
References
Allen C F H and Van Allan J A 1955Org. Syntheses Coll. ed. A H Blatt (New York: John Wiley and Sons)3 1
Angadiyavar C S and George M V 1971J. Org. Chem. 36 1589
Angadiyavar C S, Sukumaran K B and George M V 1971Tetrahedron Lett. 633
Balachandran K S 1972 Nickel peroxide oxidation of organic compounds Ph. D. Thesis, IIT, Kanpur
Balachandran K S, Hiriyakkanavar I and George M V 1975Tetrahedron 31 1171
Bamberger E and Grab J 1901Ber. 34 531
Bellamy A J and Guthrie R D 1965J. Chem. Soc. 2788
Bhatnagar I and George M V 1967J. Org. Chem. 32 2252
Biltz H 1899Ann. 305 165
Biltz H and Weiss R 1902Ber. 3519
Brown G H and Shaw W G 1961Rev. Pure Appl. Chem. 11 2
Buckingham J 1969Q. Rev. 23 37
Bunburry D L and Chuang T T 1969Can. J. Chem. 47 2045
Chalkley L 1929Chem. Rev. 6 217
Chaplin A F, Hey D H and Honeyman J 1959J. Chem. Soc. 3194
Chattaway F D 1906J. Chem. Soc. 89 462
Chernova A V, Shagidullin R R and Kitaev Y P 1967Zh. Org. Khim. 3 916;Chem. Abstr. 67 53349
Cope A C, Smith D S and Cotter R J 1963Org. Syntheses Coll. ed. N Rabjohn (New York: John Wiley)4 377
Craebe H and Gfeller E 1893Ann. 276 10
Criegee R and Lohaus G 1951Chem. Ber. 84192
Curtin D Y and Alexandrou N E 1966Tetrahedron 22 1309
El Khadem H 1963Adv. Carbohydr. Chem. 18 99
El Khadem H 1965Adv. Carbohydr. Chem. 20 139
El Khadem H and El-Shafei Z M 1958J. Chem. Soc. 3117
Exelby R and Grinter R 1965Chem. Rev. 65 247
George M V and Balachandran K S 1975Chem. Rev. 75 491
Godt H C Jr and Quinn J F 1956J. Am. Chem. Soc. 78 1461
Hauptman S, Kluge M, Seidig K D and Wilde H 1965Angew. Chem. Int. Ed. Engl. 4 688
Lewis G E and Spencer G L 1975Aust. J. Chem. 28 1733
Maier G and Heep U 1965.Angew. Chem. Int. Ed. Engl. 4 956
Maruyama K, Ono K, and Osugi J 1972Bull. Chem. Soc. Jpn. 45 847
Morrison H, Danishefsky S and Yates P 1961J. Org. Chem. 26 2617
Pausacker K H 1950J. Chem. Soc. 3478
Rinehart K L Jr 1973 inOxidation and reduction of organic compounds (New Jersey: Prentice Hall) p 131
Spasov A V, Elenkov D and Robev S 1951Bulgarska. Akad. Nauk. Otdel. Geol. Geograf. Kim. Nauk. 1 229; 1953Chem. Abstr. 47 2153
Spasov A V and Robev S 1953Bull. Inst. Chim. Acad. Bulgare. Sci. 2 3; 1955Chem. Abstr. 49 5372
Shredov V I, Altukhova L B and Griner A N 1964 USSR165 464; 1965Chem. Abstr. 62 5208
Stolle R 1926Ber. 59 1742
Sukumaran K B 1974Studies in valence isomerism in heterosystems Ph.D. Thesis, IIT, Kanpur
Sukumaran K B, Angadiyavar C S and George M V 1972Tetrahedron 28 3987
Sukumaran K B, Satish S and George M V 1974Tetrahedron 30 445
Vogel A I 1973Practical organic chemistry Third Edition (ELBS and Longman Group Ltd.) p 856
von Pechmann H 1888Ber. 21 1411 2251, 2751
von Pechmann H 1897Ber. 30 2461
Wintner C 1970Tetrahedron Lett. 2275
Wittig G and Heyn H 1964Chem. Ber. 97 1609
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhat, V., Dixit, V.M. & George, M.V. Photo-oxygenations of bisphenylhydrazones, monophenylhydrazones, bishydrazones and monohydrazones of 1,2-diketones and related systems. Proc. Indian Acad. Sci. (Chem. Sci.) 88, 223–237 (1979). https://doi.org/10.1007/BF02880929
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02880929