Abstract
Techniques of rotating-disk and catalyst were used in investigating the kinetics of dolomite dissolution in flowing CO2-H2O system. Experiments run in the solutions equilibrated with various CO2 partial pressures (PCO 2 ) from 30 to 100000 Pa. It shows that dissolution rates of dolomite are related with rotating speeds at conditions far from equilibrium. This was explained by modified diffusion boundary layer (DBL) model. In addition, the dissolution rates increase after addition of carbonic anhydrase (CA) to solutions, where the CA catalyzes CO2 conversion. However, great differences occur among various CO2 partial pressures. The experimental observations give a conclusion that the modified DBL model enables one to predict dissolution rates and their behaviour at various PCO 2 with satisfactory precision at least far from equilibrium.
Similar content being viewed by others
References
Lund, K., Fogler, H. S., McCune, C. C., Acidization—1. The dissolution of dolomite in hydrochloric acid, Chem. Eng. Sci., 1973, 28:691–700.
Herman, J. S., The dissolution kinetics of calcite, dolomite and dolomite rocks in carbon dioxide water system, PH. D. Thes., Pennsylvania State Univ., 1982.
Busenberg, E., Plummer, L. N., The kinetics of dissolution of dolomite in CO2-H2O systems at 1.5 to 65°C and 0 to 1 atm PCO 2, Amer. Jour. Sci., 1982, 282: 45–78.
Chou, L., Garreis, R. M., Wollast, R., Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals, Chem. Geol, 1989, 78: 269–282.
Liu, Z., Yuan, D., He, S. et al. Geochemical features of the geothermal CO2-water-carbonate rock system and analysis on its CO2 Sources, Science in China, Series D, 2000, 43(6): 569–576.
Shangguan, Z., Bai, C, Sun M., Mantle-derived magmatic gas releasing features at the Rehai area, Tengchong County, Yunnan Province, China, Science in China, Series D, 2000, 43(2): 132–140.
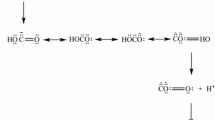
Dreybrodt, W., Lauckner, J., Liu, Z. et al., The kinetics of the reaction CO2+ H2O⇒H++ HCO3 - as one of the rate limiting steps for the dissolution of calcite in the system H2O-CO2-CaCO3, Geochim. Cosmochim. Acta,1996, 60: 3375–3381.
Liu, Z., Dreybrodt, W., Dissolution kinetics of calcium carbonate minerals in H2O-CO2 solutions in turbulent flow: the role of the diffusion boundary layer and the slow reaction H2O+CO2↔H++HCO3 - Geochim. Cosmochim. Acta,1997, 61: 2879–2889.
Dreybrodt, W., Eisenlohr, L., Madry, B. et al., Precipitation kinetics of calcite in the system CaCO3-H2O-CO2: The conversion to CO2by the slow process H++HCO3 - ⇒ CO2 + H2O as a rate limiting step, Geochim. Cosmochim. Acta,1997, 61:3897–3904.
Dreybrodt, W., Buhmann, D., A mass transfer model for dissolution and precipitation of calcite from solutions in turbulent motion, Chem. Geol., 1991, 90: 107–122.
Dreybrodt, W., Processes in Karst Systems, Springer Series in Physical Environment, Heidelberg: Springer, 1988.
Levich, V. G., Physicochemical Hydrodynamics, Englewood Ceiffs: Prentice-Hall, 1962.
Pleskov, Y V., Filinovskii, V. Y., The Rotating Disc Electrode, New York: Consultants Bureau, 1976.
Plummer, L. N., Wigley, T. M. L., Parkhurst, D. L., The kinetics of calcite dissolution in CO2-water systems at 5–60°C and 0.0-1.0 atm CO2, Am. J. Sci., 1978, 278: 179–216.
Kern, M.D., The hydration of carbon dioxide, J. Chem. Educ., 1960,37: 14–23.
Usdowski, E., Reactions and equilibria in the systems CO2-H2O and CaCO3-CO2-H2O, Areview, N. J. Mineral Abh.,1982, 144: 148–171.
Arvidson, R. S., Mackenzie, F. T., The dolomite problem: the control of precipitation kinetics by temperature and saturation state, Amer. Jour. Sci., 1999, 299: 257–288.
Gautelier, M., Oelkers, E. H., Schott, J., An experimental study of dolomite dissolution rates as a function of pH from-0.5 to5 and temperature from 25to80°C,Chem. Geol., 1999, 157: 13–26.
Herman, J. S., White, W. B., Dissolution kinetics of dolomite: effect of lithology and fluid velocity, Geochim. Cosmochim. Acta,1985,49: 2017–2026.
Orton, R., Unwin, P. R., Dolomite dissolution kinetics at low pH: a channel-flow study, J. Chem. Soc, Faraday Trans., 1993, 89:3947–3954
Vasconcelos, C. O., McKenzie, J. A., Bernasconi, S. et al., Microbial mediation as a possible mechanism for natural dolomite formation at low temperature, Nature, 1995, 337: 220–222.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Wolfgang, D. Kinetics and rate-limiting mechanisms of dolomite dissolution at various CO2 partial pressures. Sc. China Ser. B-Chem. 44, 500–509 (2001). https://doi.org/10.1007/BF02880680
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02880680