Abstract
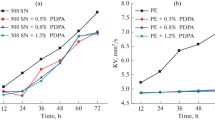
Three kinds of the antioxidation and anticorrosion additives from the N-substituted perfluoropolyalkylether phenylamide (PFPEA) were selected and synthesized. UV and IR spectral analyses were carried out, and strong absorption peaks of UV from benzene ring are about 240.7, 215.4 and 230.1 nm, respectively. The characteristic peaks of IR from the C=O are about 1713.9, 1712.2 and 1710.8 cm-1, respectively. The antioxidant and anticorrosive property was tested for the three synthesized additives. The results show that the weight loss of lubrication oil can decrease by 1/7, 1/9 and 1/25 respectively after adding synthesized additives. The thermal decomposition temperature(TD) in the presence of Al2O3 can increase by 19–22°C. From theoretic and experimental study it indicates that the PFPEAs with nitrogen heteroatom not only accepts electron from perfluoropolyalkylether oxygen radical (RfO.) to form a stable adduct and to prevent RfO. decomposing further, but also donates electron to form chemical adsorption film and to protect metal from corrosion. These additives have shown the better property of the antioxidation and anticorrosion. An electron-releasing group, or phenyl group, introduced to the N-atom of this kind of compound can improve the antioxidant and anticorrosive efficiency of the additives.
Similar content being viewed by others
References
Wang Daxi, Feng Ying, Action mechanism of antioxidation and anticorrosion and molecular design for perfluoropolyether fluid additives (I)—Action mechanism of additive and property of donating-accepting electron, Science in China, Series B, 2001, 44(4): 427–435.
Lois, J. G, Carl, E. S., High temperature oxidative stability of C3F7O[CF(CF3)CF2O]xC3F7 and CF3(OCFCF2CF2)7CF3per- fluoropolyalkylethers and formulation in the presence of metals, Lubrication Engineering, 2000, 56(1): 17–22.
Snyner, C. E., Gschwender, L. J., Scott, O. L., Characterization of model perfluoropolyalkyl ethers by miniaturized thermal oxidative techniques—Part II: Pressure differential scanning calorimetry, Tribology Transactions, 1995, 38(3): 733- 737.
Helmick, L. S., Jones, W. R., Determination of the thermal stability of perfluoropolyalkylethersby tensimetry, Lubrication Engineering, 1994, 50(6): 449–457.
Paciorek K. J. L., Kratzer, R. H., Stability of perfluoropolyalkyl ethers, Journal of Fluorine Chemistry, 1994, 67: 169–175.
Paciorek, K. J. L., Masuda, S. R., Lin, W. -H., Perfluoroalkyl- and perfluoropolyalkylether-substituted quinoxalines, Journal of Fluorine Chemistry, 1996, 78: 27–38.
Chen, G. J., Chen, L. S., New mehtods for the preparation of perfluoroalkyl- and perfluoroalkylether-s-triazines, Journal of Fluorine Chemistry, 1998, 89: 217–221.
Wang Quan-Fu, Mao Yun-Yu, Zhu Shi-Zheng, A novel synthesis of 2-per(poly)fluoroalkyl-lH-benzimidazoles of 2-per(poly)fluoroalkyl benzothiazoles, Journal of Fluorine Chemistry, 1999, 95: 141–143.
Boiko, V. N., Shchupak, G. M., Ion-radical perfluoroalkylation, Part II. Perfluoroalkylation of thiols by perfluoroalkyl iodides in the absence of initiators, Journal of Fluorine Chemistry, 1994, 69: 207–212.
Smart, B. E., Dixon, D. A., Heterolytic C-F bond energies and stabilities of poly(perfluoroethers), Journal of Fluorine Chemistry, 1992, 57: 251–258.
Paciorek, K. J. L., Masuda, S. R., Lin, W. -H. et al., Thermal oxidative stability of perfluoropoly-alkyl ether and development of quantitative structure-stability relationships, Journal of Fluorine Chemistry, 1996, 76: 21–27.
Demers, J. R., Gschqerder, L. J., A new technique for the investigation of liquid luricant degradation: The oxidation corrosion conductivity test, Lubrication Engineering, 1995, 51(4): 321–327.
Gschwender, L. J., Deorge, W. F., Soluble additives for perfluoropolyalkyl ether liquid lubricants, Lubrication Engineering, 1993, 49(9): 702–708.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, D., Li, S. & Feng, Y. Action mechanism of antioxidation and anticorrosion and molecular design for perfluoropolyether fluid additives (II). Sc. China Ser. B-Chem. 44, 486–492 (2001). https://doi.org/10.1007/BF02880678
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02880678