Abstract
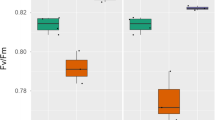
Leaves of the two new chlorophyllb-less rice mutants VG28-1, VG30-5 and the wild type rice cv. Zhonghua 11 were subjected to temperatures 28, 36, 40, 44 and 48°C in the dark for 30 min or gradually elevated temperature from 30°C to 80°C at 0.5°C/min. The thermostability of photosynthetic apparatus was estimated by the changes in chlorophyll fluorescence parameters, photosynthetic rate and pigment content, chloroplast ultrastructure and tissue location of H2O2 accumulation. There were different patterns of Fo-temperature curves between the Chlb-less mutants and the wild type plant, and the temperature of Fo rising threshold was shifted 3°C lower in the Chlb-less mutants (48°C) than in the wild type (51°C). At temperature up to about 45°C, chloroplasts were swollen and thylakoid grana became misty accompanied with the complete loss of photosynthetic oxygen evolution in the two Chlb-less mutants, but chloroplast ultrastructure in the wild type showed no obvious alteration. After 55°C exposure, the disordered thylakoid and significant H2O2 accumulation in leaves were found in the two Chlb-less mutants, whereas in the wild type plant, less H2O2 was accumulated and the swollen thylakoid still maintained a certain extent of stacking. A large extent of the changes in qP, NPQ and Fv/Fm was consistent with the Pn decreasing rate in the Chlb-less mutants during high temperature treatment as compared with the wild type. The results indicated that the Chlb-less mutants showed a tendency for higher thermosensitivity, and loss of Chlb in LHC II could lead to less thermostability of PSII structure and function. Heat damage to photosynthetic apparatus might be partially attributed to the internal oxidative stress produced at severely high temperature.
Similar content being viewed by others
References
Georgieva, K., Yordanov, I., Temperature dependence of chlorophyll fluorescence parameters of pea seedlings, J. Plant Physiol., 1993, 142: 151–155.
Havaux, M., Characterization of thermal damage to the photosynthetic electron transport system in potato leaves, Plant Sci., 1993, 94: 19–33.
Knaetzel, J., Simpson, D., Expression and organization of antenna proteins in the light- and temperature-sensitive barley mutant chlarina-104, Planta., 1991, 185: 111–123.
Bakhov, N. G., Sabat, S. C., Mohanty, P., Analysis of chlorophyll a fluorescence changes in weak light in heat treatedAmaranthus chloroplasts, Photosynth. Res., 1990, 23: 81–87.
Schreiber, U., Berry, J. A., Heat-induced changes of chlorophyll fluorescence in intact leaves correlated with damage of the photosynthetic apparatus, Planta., 1977, 136: 233–238.
Singh, A. K., Singhal, G. S., Specific degradation of D1 protein during exposure of thylakoid membranes to high temperature in dark, Photosynthetica., 1999, 36: 433–440.
Yang, C. M., Osterman, J. C., Markwell, J., Temperature sensitivity as a general phenomenon in a collection of chlorophyll-deficient mutants of sweetclover (Melilotus alba), Biochem. Gen., 1990, 28: 31–40.
Havaux, M., Tardy, F., Thermostability and photostability of photosystem II in leaves of the chlorina-f2 barley mutant deficient in light-harvesting chlorophylla/b protein complex, Plant Physiol., 1997, 113: 913–923.
Wang, J., Lin, L., Wan, X. S. et al., Generation and molecular analysis of a population of transgenic rice plants carrying Ds element, Acta Phytophysiol. Sin., 2000, 26: 501–506.
Lin, Z. F., Peng, C. L., Lin, G. Z. et al., Photosynthetic characteristics of two new chlorophyll b-less rice mutants, Photosynthetica, 2003, 41: 61–67.
Lin, Z. F., Lin, G. Z., Peng, C. L. et al., Alteration of chlorophyll-protein complex components and distribution of excitation energy between two photosystems in two new rice chlorophyll b-less mutants, Photosynthetica, 2003, 41: 589–595.
Arnon, D. L., Copper enzymes in isolated chloroplast: Polyphenoloxidase inBeta vulgaris, Plant Physiol., 1949, 24: 1–15.
Maxwell, K., Johnson, G. N., Chlorophyll fluorescence-a practical guide, J. Exp. Bot., 2000, 345: 659–668.
Laisk, A., Oja, V., Rasulov, B. et al., Quantum yields and rate constants of photochemical and nonphotochemical excitation quenching, Plant Physiol., 1997, 115: 803–813.
van Kooten, O., Snel, J. F. H., The use of chlorophyll fluorescence nomenclature in plant stress physiology, Photosynth. Res., 1990, 25: 147–150.
Ganesan, V., Thomas, G., Salicylic acid response in rice: Influence of salicylic acid on H2O2 accumulation and oxidative stress, Plant Sci., 2001, 160: 1095–1106.
Gounaris, K., Brain, A. P. R., Quinn, P. J. et al., Structural reorganization of chloroplast thylakoid membranes in response to heat stress, Biochim. Biophys. Acta., 1984, 766: 198–208.
Tan, X. X., Xu, D. Q., Shen, Y. K., Both spillover and light absorption cross-section changes are involved in the regulation of excitation energy distribution between the two photosystems during state transitions in wheat leaf, Photosynth. Res., 1998, 56: 95–102.
Vani, B., Saradhi, P. P., Mohanty, P., Alteration in chloroplast structure and thylakoid membrane composition due toin vivo heat treatment of rice seedlings: Correlation with the functional changes, J. Plant Physiol., 2001, 158: 583–592.
Georgieva, K., Fedina, I., Maslenkova, L. et al., Response of chlorina barley mutants to heat stress under low and high light, Funct. Plant Biol., 2003, 30: 515–524.
Ducruet, J. M., Relation between the heat-induct increase of Fo fluorescence and a shift in the electronic equilibrium at the acceptor side of photosystem 2, Photosynthetica., 1999, 37: 335–338.
Mishra, R. K., Singhal, G. S., Function of photosynthetic apparatus of intact wheat leaves under high light and heat stress and its relationship with peroxidation of thylakoid lipids, Plant Physiol., 1992, 98: 1–6.
Li, G. F., Mao, H. B., Ruan, X. et al., Association of heat-induced conformational change with activity loss of Rubisco, Biochem. Biophys. Res. Commun., 2002, 290: 1128–1132.
Elrad, D., Niyogi, K. K., Grossman, A. R., A major light harvesting polypeptide of photosystem II function in thermal dissipation, Plant Cell., 2002, 14: 1801–1816.
Andrews, J. R., Fryer, M. J., Baker, N. R., Consequences of LHC II deficiency for photosynthetic regulation in chlorina mutants of barley, Photosynth. Res., 1995, 44: 81–91.
Breusegem, F. V., Vranová, E., Dat, J. F. et al., The role of active oxygen species in plant signal transduction, Plant Sci., 2001, 161: 405–414.
Foyer, C. H., Lopez-Delgado, H., Dat, J. F., Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling, Physiol. Plant., 1997, 100: 241–254.
Dat, J. F., Lopez-Delgado, H., Foyer, C. H. et al., Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings, Plant Physiol., 1998, 116: 1351–1357.
Kang, H. M., Saltveit, M. E., Antioxidant enzymes and DPPH-radical scavenging activity in chilled and heat-shocked rice (Oryza sativa L.) seedlings radicles, J. Agric. Food Chem., 2002, 50: 513–518.
Pellinen, R. I., Korhonen, M.-S., Tauriainen, A. A. et al., Hydrogen peroxide activates cell death and defense gene expression in birch, Plant Physiol., 2002, 130: 549–560.
Lin, Z. F., Li, S. S., Lin, G. Z. et al., The accumulation of hydrogen peroxide in senescing leaves and chloroplasts in relation to lipid peroxidation, Acta Phytophysiol Sin., 1988, 14: 16–22.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, Z., Peng, C., Xu, X. et al. Thermostability of photosynthesis in two new chlorophyllb-less rice mutants. Sci. China Ser. C.-Life Sci. 48, 139–147 (2005). https://doi.org/10.1007/BF02879666
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02879666