Abstract
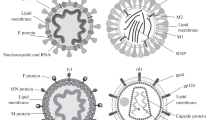
The first Raman spectra of HIV1-HIV2 in human sera and hypericin-induced photosensitive damage of the virus have been obtained. The prominent Raman lines in the spectra are assigned respectively to the carbohydrates of viral glycoprotein, RNA, protein and lipid. The spectra are dominated by Raman scattering of the carbohydrates. The lines of D-Mannose and N-acetylglucosamine in carbohydrates are obvious and there is a β-configuration in the anomeric C1 position in D-Mannose. The viral RNA duplexes bound assumes an A-form geometry. The lines of backbone phosphate group, bases (involving interbase hydrogen bonding) and ribose of the RNA are complete and distinct. The secondary structure of the viral protein maintains α-helix, β-sheet, β-turn and random coil. Its side chains are rich and vary from tryptophan, phenylalanine and “buried” tyrosine; the stable conformation of the S-S bond of gauche-gauche-gauche; the two forms of C-S bonds of gauche and trans; to sulfhydrl group and ionized and unionized carboxyl groups. The viral lipid bilayer molecules are probably in the liquid ordered phase or the gel phase. It was observed that the hypericin-induced photosensitive damage of HIV1-HIV2 in human sera changed various components of HIV1-HIV2 in different degrees: The orderly A-form viral RNA would become a disordered viral RNA. There were a breakage of interbase hydrogen bonds and disruption of vertical base-base stacking interactions. In addition, the groups of ribos and four bases were damaged obviously. A decrease in ordered structure (α-helix and β-sheet) of viral protein is accompanied by an increase in random coil. The Tyr buried in the three-dimensional structure of protein was damaged, but it was still “buried” and the damage of C-S bond of trans form was stronger. The groups of carbohydrates, including D-Mannos and N-acetyl glucosamine, in viral envelope glycoprotein had also been changed. The hydrophilic C-N bond of choline in viral lipid was damaged, which was the possible binding site to hypericin, whereas the viral lipids bilayers were still probably in the liquid ordered phase or the gel phase. So the space structure of HIV1-HIV2 was damaged under the experimental conditions, which might block viral infection and inhibit its growth and breeding. It is apparent that the laser Raman spectra have provided certain direct evidence at the molecular level for photosensitive damage of HIV1-HIV2.
Similar content being viewed by others
References
Thomas, G. J. Jr., Applications of Raman spectroscopy in structural studies of viruses, nucleoproteins and their constituents, in Spectroscopy of Biological Systems (eds. Clark, R. J. H., Hester, R. E.), Vol. 13, New York: John Wiley & Sons, 1986, 233–303.
Thomas, G. J. Jr., Viruses and Nucleoproteins, in Biological Applications of Raman Spectroscopy (ed. Spiro, T. G.), Vol. 1, New York: John Wiley & Sons, 1987, 135–201.
Xu, Y. M., Lu, C. Z., Raman spectroscopic study on human immunodeficiency virus (HIV1-HIV2) space structure and microcosmic and photosensitive damage to the virus, Proceedings of the XVth Inteernational Conference on Raman Spectroscopy (eds. Asher, S. A., Stein, P.), August 11–16, 1996, Pittsburgh, PA, USA, New York: John Wiley & Sons, Ltd., 490–491.
Coffin, J., Ashley, H., Jay, A. et al., What to call the AIDS virus? Nature, 1986, 321: 10.
Weiss, R. A., How does HIV cause AIDS ? Science, 1993, 260: 1273–1279.
Gallo, R. C., Montagnier, L., AIDS in 1988, Sci. Am., 1988, 259: 25–32.
Greene, W. C., AIDS and the immune system, Sci. Am., 1993, 269: 67–73.
Haseltine, W. A., Wong, S. F., The molecular biology of the AIDS virus, Sci. Am., 1988, 259: 34–42.
McDougal, J. S., Kennedy, M. S., Sligh, J. M. et al., Binding of HTLV-III/LAV to T4+ T cell by a complex of the 110 K viral protein, Science, 1986, 31: 382–385.
Klatzmann, D., Champagne, E., Chamaret, S. et al., T-lymphocyte T4 molecule behaves as the resepto for human retrovirus, Nature, 1984, 312: 767–768.
Greene, W. C., The molecular biology of human imunodeficincy virus type 1 infection, N.E.J. Med., 1991, 324: 308–317.
Pantaleo, G., Graziosi, C., Demarest, J. F. et al., HIV infection is active and pogressive in lymphoid tissue during the clinically latent stage of disease, Nature, 1993, 362: 355–358.
Feinberg, M. B., Greene, W. C., Molecular insights into human immunodeficiency virus type-1 pathogenesis, Curr. Opin. in Immun., 1992, 4: 466–474.
Cheng, Li, in Medical and Molecular Virology (ed. Wen, Y. M.), Beijing: People’s and Medical Press, 1990, 193–202.
Wang, X. Z., Medical Virology Basis, Fundamental Techniques & Methods, Beijing: Academy Press, China, 1987, 34–44.
Feizi, T., Larkin, M., AIDS and glycosylation, Glycobiology, 1990, 1: 17–23.
Chau, S. N., Ho, D. D., Sun, C. R. Y. et al., Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120, J. Virol., 1989, 63: 3579–3585.
Richards, A. D., Phylip, L. H., Farmerie, W. G. et al., Sensitive, soluble chromogenic substrates for HIV-1 protease, J. Biol. Chem., 1990, 265: 7733–7736.
Cohen, J., Weiee, R. A., Bloom, B. R. et al., The new face of AIDS, Science, 1996, 272: 1855–1890.
Ho, D. D., Kaplan, J. C., Rackauskas, I. E. et al., Second conserved domain of gp120 is important for HIV infectivity and antibody neutralization, Science, 1988, 239: 1021–1023.
Rusche, J. R., Javaherian, K., McDanal, C. et al., Antibodies that inhibit fusion of human immunodeficiency virus-ifected cells bind a 24-amino acid sequence of the viral envelope, gp120, Proc. Natl. Acad. Sci., USA, 1988, 85: 3198–3202.
Kowalski, M., Potz, J., Basiripour, L. et al., Functional regions of the envelope glycoprotein of human immunodeficicncy virus type 1, Science, 1987, 237: 1351–1355.
Gelderblom, H. R., Hausmann, E. H. S., Ozel, M. et al., Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins, Virology, 1987, 156: 171–176.
Veronese, F. M., DeVico, A. L., Copeiand, T. D. et al., Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene, Science, 1985, 229: 1402–1405.
Darke, P. L., Leu, C. T., Davis, L. J., Human immunodeficiency virus protease, J. Biol. Chem., 1989, 264: 2307–2312.
Larder, R. A., Purifoy, D. J. M., Powell, K. L. et al., Structural studies of the acquired immunodeficiency syndrome virus reverse transcriptase, Am. J. Med., 1988, 85: 173–175.
Hartman, K. A., Clayton, N., Thomas, G. J. Jr., Studies of virus structure by Raman spectroscopy I. R17 virus and R17 RNA, Biochem. Biophys. Res. Commun., 1973, 50: 942–949.
Thomas, G. J. Jr., Prescott, B., Studies of virus structure by laser-Raman spectroscopy (II): MS2 phage, MS2 capsids and MS2 RNA in aqueous solutions, J. Mol. Biol., 1976, 102: 103–124.
Frankel, A. D., Young, J. A. T., HIV-1: Fifteen Proteins and an RNA, Annu. Rev. Biochem., 1998, 67: 1–25.
Marx, J. L., AIDS drugs — coming but not have, Science, 1989, 244: 287.
Holden, C., Treating AIDS with worts, Science, 1991, 25: 522.
Schinazi, R. F., Chu, C. K., Babu, J. R. et al., Antraquionones as a class of antiviral agents against human immunodeficiency virus, Antiviral Res., 1990, 13: 265–272.
Takahashi, I., Nakanishi, S., Kobayashi, E. et al., Hypericin and pseudohypericin specifically inhibit protein kinase C: Possible relation to their anti-retroviral activity, Biochem. Biophys. Res. Commun., 1989, 165: 1207–1212.
Diwu, Z., Zimmlermaun, J., Meyer, T. et al., Design, synthesis and investigation of mechanisms of action of novel protein kinase C inhibitors: Perylenequinonoid pigments, Biochem. Pharmacol., 1994, 47: 373–385.
Lenard, J., Rabson, A., Stevenson, N. R. et al., Photodynamic inactivation of viral fusion and infectivity by hypericin and rose bengal: Effects on HIV and other enveloped virus, J. Cell. Biochem., 1993, 17 E-F, 17.
Lenard, J., Rabson, A., Vanderoef, R., photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped virus using hypericin and rose Bengal: Inhibition of fusion and syncytia formation, Proc. Natl. Sci. USA, 1993, 90: 158–162.
Fields, A. P., Bednarik, D. P., Hess, A. et al., Human immunodeficiency virus induces phosphorylation of its cell surface receptor, Nature, 1988, 333: 278–280.
Degar, S., Prince, A. M., Pascual, D. et al., Inactivation of the human immunodeficiency virus by hypericin: evidence for photochemical alterations of p24 and ablock in uncoating, AIDS Res. Hum. Retroviruses, 1992, 8: 1929–1936.
Meruelo, D., Lavie, G., Lavie, D., Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin, Proc. Natl. Acad. Sci. USA, 1988 85: 5230–5234.
Diwu, Zhenjun, Novel therapeutic and diagnostic applications of hypocrellins and hypericins, Photochem. and Photobiol., 1995, 61: 529–539.
Lopez-Bazzocch, I., Hudson, J. B., Towers, G. H. N., Antiviral activity of the photoactive plant pigment hypericin, Photochem. and Photobiol., 1991, 54: 95–98.
Hudson, J. B., Lmperial, V., Haugland, R.P., Diwu, Z., Antiviral activities of photoactive perylenequinones, Photochemistry and Photobiology, 1997, 65: 352–354.
Hudson, J. B., Harris, L., Towers, G. H. N., The importance of light in the anti-HIV effect of hypericin, Antivival Res., 1993, 20: 173–178.
Meruelo, D., Prince, A. M., Pascual, D. et al., The potential use of hypericin as inactivator of retroviruses and other viruses in blood products, Blood, 1993, 82: 205A.
Dougherty, T. J., Photodynamic therapy, Photochemistry and Photobiology, 1993, 58: 895–900.
Lavie, G. F., Valentine, G., Levin, Y. et al., Studies of the mechanisms on action of the antiretroviral agent hypericin and pseudohypericin, Proc. Natl. Acad. Sci. USA, 1989, 86: 5963–5967.
Lord, R. C., Yu, N. T., Laser-Raman spectroscopy of biomolecules (I): Native lysozyme and its constituent amino acids, J. Mol. Biol., 1970, 50: 509–524.
Carey, P. R., Biochemical Applications of Raman and Resonance Raman Spectroscopies, New York: Harcout Brace Jovanovich, 1982, 71–98.
Zhang, Z. Y., Xu, Y. M., The Molecular of photoporphyrin (YHPD)’s photosensitization, Science in China, Ser. B, 1992, 35: 437–444.
Koenig, J. L., Infrared and Raman Spectroscopy of Biological Molecules (ed. Theophanides, M. T.), Holland: NATO Scientific Affairs Division, 1979, 109–125, 125–137.
Parker, F. S., Applications of Infrared, Raman, and Resonance Raman Spectroscopy in Biochemistry, New York: Plenum Press, 1983, 315–347.
Zhao, H., Xu, Y. M., Lu, C. Z., Raman spectroscopic study of D-Mannose after the photosensitive damage caused by hypericin, Int. Asian J. of Spctroscopy, 1997, 1: 71–76.
Krimm, S., Bandekar, J., Vibrational analysis of peptides, polypeptides, and proteins V, Normal vibrations of ß-turn, Biopolymers, 1980, 19: 1–29.
Bandekar, J., Krimm, S., Vibrational analysis of peptides, polypeptides, and proteins (VI): Assignment of β-turn modes in insulin and other proteins, Biopolymers, 1980, 19: 31–36.
Thomas, G. J. Jr., Kyogoku Yoshimasa, Biological Science, in Infrared and Raman Spectroscopy, Part C (eds. Bram, E. G. Jr. et al.), New York and Besel: Marcel Dekker, Inc., 1977, 778–782
Tu, A. T., Raman Spectroscopy in Biology: Principles and Applications, New York: John Wiley and Sons, Inc., 1982, 187–205.
Xu, Y. M., Zhao, H. X., Zhang, Z. Y., Raman spectroscopic study of microcosmic and photosensitive damage on the liposomes of the mixed phospholipids sensitized by hypocrellin and its derivatives, Int. J. Photochem. & Photobiol. B: Biol., 1998, 43: 41–46
Xu, Y. M., Zhang, Z. Y., Zhang, W., Raman spectroscopic characteristics of microcosmic and photosensitive damage on space structure of liposomes sensitized by hypocrellin and its derivatives, Science in China, Ser. C, 1998, 41(5): 460–464.
Xu, Y. M., Yang, H.Y., Zhing, Z. Y., Raman spectroscopic study of space structure of membrane proteins and membrane lipids in photodamaged human erythrocyte sensitized by hypocrellin B, Science in China, Ser. C, 1998, 41(7): 608–616.
Brown, D. A., London, E., Structure and origin of ordered lipid domains on biological membranes, J. Membrane Biol., 1998, 164: 103–114.
Brunner, H., Sussner, H., Raman scattering of native and thermally denatured lysozyme, Biochim. Biophys. Acta, 1972, 271: 16–22.
Xu, Y. M., Zhang, Z. Y., Zhao, K. J. et al., Molecular mechanism of high energy proton radiation—Raman spectroscopic character of mirocosmic damage in the space structure of DNA, Science in China, Ser. B, 1993, 36: 1325–1332.
She, C. Y., Dinh, N. D., Tu, A. T., Laser Raman scattering of glucosamine, N-acetylglucosamine and glucuronic acid, Biochim. Biophys. Acta, 1974, 372: 345–355.
Kuwabara, M., Zhang, Z. Y., Yosaii, G., E.S.R.of spin trapped radicals in aqueous solutions of Pyrimidine nucleosides and nucleotides, Reaction of the Hydroxyl Radicals Int. J. Radiat, Biol., 1982, 41: 241–259.
Erfurth, S. C., Peticolas, W. L., Melting and premelting phenomenon in DNA by Laser Raman scattering, Biopolymer, 1975, 14: 247–264.
Gaber, B. P., Peticalas, W. L., On the quantitative interpretation of biomembrane structure by Raman spectroscopy, Biochim. Biophys. Acta, 1977, 465: 260–274.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Y., Lu, C. Raman spectroscopic study on structure of human immunodeficiency virus (HIV) and hypericin-induced photosensitive damage of HIV. Sci. China Ser. C.-Life Sci. 48, 117–132 (2005). https://doi.org/10.1007/BF02879664
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02879664