Abstract
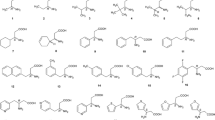
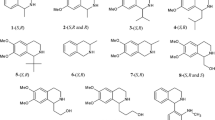
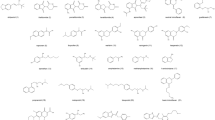
In normal phase condition, a series of chiral phosphorus organic compounds have been separated by high-performance liquid chromatography. In order to study the retention and chiral recognition mechanism, the method of quantitative structure-enantioselectivity retention relationships (QSERRs) has been investigated from the quantitative equations established between the chromatographic retention of enantiomers and their molecular descriptors of physicochemical properties. The results show that on the Pirkle-type chiral stationary phase (CSP) of Sumichiral OA4700, it is the parameter of LUMO that gives the most contribution to the chromatographic retention of O-ethyl O-(substituted) phenyl N-isopropyl phosphoroamidothioates resulting from the interaction of hydrogen bond and (or) π-π interaction. Meanwhile, the chiral recognition is formed from the contribution of logP and LUMO.
Similar content being viewed by others
References
Armstrong, D. W., Optical isomer separation by liquid chromatography, Anal. Chem., 1987, 59(2): 84A-91A.
Davankov, V. A., Kurganov, A. A., Bochkov, A. S., Advances in Chromatography, New York: Marcel Dekker, 1983.
Pirkle, W. H., Pochapsky, T. C., Considerations of chiral recognition relevant to the liquid chromatographic separation of enantiomers, Chem. Rev., 1989, 89: 347–362.
Meng, Z. H., Wang, Q. H., Zhu, D. Q. et al., Application of molecular imprinting in chiral separation, Chinese J. Anal. Chem., 1997, 25(3): 349–354.
Wainer, I. W., A Practical Guide to the Selection and Use of HPLC Chiral Stationary Phase (ed. Baker, J. T.), New York: Phillipsburg NJ, 1988.
Berthod, A., Chang, S. C., Armstrong, D. W., Empirical procedure that uses molecular structure to predict enantioselectivity of chiral stationary phases, Anal. Chem., 1992, 64: 395–404.
Norinder, U., Hermansson, J., Chiral separation of N-aminoalkylsuccinimides on an α1-acid glycoprotein column: a quantitative structure-enantioselectivity relationship study, Chirality, 1991, 3(5): 422–426.
Topiol, S., Sabio, M., Moroz, J. et al., Computational studies of the interactions of chiral molecules: complexes of methyl N-(2-naphthyl) alaninate with N-(3,5-dinitrobenzoyl) leucine n-propylamide as a model for chiral stationary phase interactions, J. Am. Chem. Soc., 1988, 110: 8367–8376.
Hansch, C., Fujita, T., ρ-σ-π analysis, a method for the correlation of biological activity and chemical structure, J. Am. Chem. Soc., 1964, 86: 1616–1626.
Wang, L. S., Zhi, Z. L., Gao, S. T., Molecular Structure and Chromatographic Retention, Beijing: Chemical Industry Press, 1994.
de Julián-Ortiz, J. V., Garcia-Domenech, R., Gálvez Alvarez, J. et al., Use of topological descriptors in chromatographic chiral separations, J. Chromatogr. A, 1996, 719: 37–44.
Booth, T. D., Wainer, I. W., Investigation of the enantioselective separations of α-alkylarylcarboxylic acids on an amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase using quantitative structure-enantioselective retention relationships, Identification of a conformationally driven chiral recognition mechanism, J. Chromatogr. A, 1996, 737: 157–169.
Kaliszan, R., Noctor, T. A. G., Wainer, I. W., Quantitative structure-enantioselective retention relationships for the chromatography of 1,4-benzodiazepines on a human serum albumin based HPLC chiral stationary phase: an approach to the computational prediction of retention and enantioselectivity, Chromatographia, 1992, 33(11/12): 546–550.
Booth, T. D., Wainer, I. W., Mechanistic investigation into the enantioselective separation of mexiletine and related compounds, chromatographed on an amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase, J. Chromatogr. A, 1996, 741: 205–211.
Kaliszan, R., Kaliszan, A., Noctor, T. A. G. et al., Mechanism of retention of benzodiazepines in affinity, reversed-phase and adsorption high-performance liquid chromatography in view of quantitative structure-retention relationships, J. Chromatogr., 1992, 609: 69–81.
Booth, T. D., Azzaoui, K., Wainer, I. W., Prediction of chiral chromatographic separations using combined multivariate regression and neural networks, Anal. Chem., 1997, 69: 3879–3883.
Chen, H., Lu, X. Y., Gao, R. Y. et al., Investigation of retention and chiral recognition mechanism using quantitative structure-enantioselectivity retention relationship in high performance liquid chromatography, Chin. J. Chem., 2000, 18(2): 194–197.
Chen, H., Lu, X. Y., Huang, J. M. et al., Investigation of retention and chiral recognition mechanism of the derivative β-cyclodextrin bonded stationary phase (I), Chem. J. Chinese Univ., 2000, 21(4): 562–565.
Chen, H., Lu, X. Y., Gao, R. Y. et al., Influence of temperature and flow rate on enantioselectivity about derivative β-cyclodextrin bonded stationary phase in normal-phase liquid chromatography, Chem. J. Chinese Univ., 2000, 21(2): 233–236.
Chen, R. Y., Yang, H. Z., Zhang, Y. J. et al., The synthesis of phosphoramidothioate compounds and quantitative correlation of structure-herbicidal activity, Science in China, Series B, 1985, 28(5): 394–400.
Chen, R. Y., Yang, H. Z., Zeng, Q. et al., The synthesis of alkyl aryl N-alkylthionophosphoramidates and quantitative correlation of structure-activity, Acta Chimica Sinica, 1981, 39(7): 644–651.
Chen, R. Y., Yang, H. Z., Xing, X. D. et al., Studies on the ortho effects in quantitative structure-herbicidal activity relationship of O-aryl O-ethyl N-isopropylphosphoramidothioates, Chem. J. Chinese Univ., 1985, 6(10): 897–902.
Yang, H. Z., Zhang, Y. J., Wang, L. X. et al., Quantitative structure-activity study of herbicidal O-aryl O-ethyl N-isopropylphosphoramidothioates, Pesticide Biochemistry and Physiology, 1986, 26: 275–283.
Kaliszan, R., Quantitative Structure-Chromatographic Retention Relationship, New York: John Wiley & Son, 1987.
Azzaoui, K., Morin-Allory, L., Comparison and quantification of chromatographic retention mechanisms on three stationary phase using structure-retention relationships, Chromatographia, 1996, 42(7/8): 389–395.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Chen, H., Gao, R. et al. Retention and chiral recognition mechanism of organo-phosphorus compounds in high-performance liquid chromatography. Sc. China Ser. B-Chem. 44, 147–153 (2001). https://doi.org/10.1007/BF02879532
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02879532