Abstract
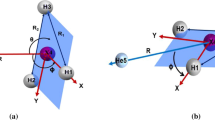
The intermolecular potential energy surface of He-LiH complex was studied using the full-electronic complete forth-order Mαller-Plesset perturbation (MPPT) method. Inab initio calculations, the bond length of LiH was fixed at 0.159 5 nm The potential has two local minima of Vm= - 179.93 cm-1 for the linear He-LiH geometry atRm = 0.227 nm and Vm = - 10.44 cm- 1 for the linear He-HLi geometry atRm = 0. 516 nm. The potenial exhibits strong anisotropy. The analytic potential function with 31 parameters was determined by fitting to the calculatedab inttio potentials. The influence of variation of LiH bond length on the potential energy surface was also studied.
Similar content being viewed by others
References
Matiland, G. C., Rigby, M., Smith, E. B.et al., Intermolecular Forces: Their Origin and Determination, Oxford: Clarendon Press, 1981.
Chalasinski, G., Szczesniak, M., Origins of structure and energetics of van der Waals clusters fromab initio calculations,Chemical Reviews, 1994, 94: 1723.
Silver, D. M., Rotationally inelastic collisions of LiH with He (I):ab initio potential energy surfaces,J. Chem. Phys., 1980, 72: 6445.
Matias, M. A., Raimondi, M., Tornaghi, E.et al., Spin coupled valence bond theory of van der Waals systems: application to LiHHe,Mol. Phys., 1994, 83: 89.
Tao, F., Klemperer, W., Accurateab initio potential energy of Ar-HF, Ar-H2O and Ar-NH3,J. Chem. Phys., 1994, 101: 1129.
Roos, B. O., Sadlej, A., Polarized basis sets for accurate predictions of molecular electric properties: Electric moments of the LiH molecule,J. Chenl. Phys., 1985, 94: 43.
Huston, J. M., The intermolecular potential of Ar-HC1: Determination from high-resolution spectroscopy,J. Chem. Phys., 1988, 89: 4550.
Boys, S. F., Bernardi, F., The calculation of small molecular interactions by the differences of separate total energies: Some procedures with reduced errors,Mol. Phys., 1970, 19: 553.
Gaussian 92/DFT, Revision G.3, Frisch,M.J., Trucks,G.W., Schlegel, H. B., Gill, P. M. W., Johnson, B. G., Wong, M.W., Foresman, J.B., Robb, M.A., Head-Gordon, M., Replogle,E.S., Gomperts,R., Andres,J.L. Raghavachari, K., Binkley,J.S., Gonzalez,C., Martin, R.L.,Fox, D.J., Defrees, D. J., Baker,J., Stewart, J.J. P., and Pople, J. A., Gaussian, Inc., Pittsburgh PA. 1993.
Gray, C. G., Gubbins, K. E.,Theory of Molecular Fluids, Vol. 1,Fundamentals, Oxford: Clarendon Press, 1984.
Tang, K. T., Toennies, J. P., An improved simple model for the van der Waals potential based on universal damping functions for the dispersion coefficients,J. Chem. Phys., 1984, 80: 3726.
Huston, J. M., Intermolecular forces from the spectroscopy of van der Waals molecules,Annu.Rev. Phys. Chem., 1990, 41: 123.
Author information
Authors and Affiliations
Additional information
Project supported by the National Natural Science Foundation of China (No. 29673029)
Rights and permissions
About this article
Cite this article
Yan, G., Yang, M. & Xie, D. Ab initio intermolecular potential energy surface of He-LiH. Sc. China Ser. B-Chem. 40, 554–560 (1997). https://doi.org/10.1007/BF02875427
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02875427