Abstract
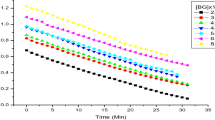
1,3. 3-trimethyl-spiro[indolino-2, 3′-[2H]naphtho[2, 1-b] [1, 4]oxazine(SP1) and 1, 3, 3-trimenthy l-9′-methoxy-spiro[indolino-2, 3′-[2H]naphtho[2, 1-b] [1, 4]oxazine] (SP2) react with hydrochloric acid to form the complex SP. HCI in isopropanol solution at room temperature. The absorption maxima of these complexes are 440 and 463 nm respectively. In acidic media, the opening form of spirooxazine can react with hydrochloric acid to form the complex PMC. HCl via zwitterion form. The absorption spectra of PMC. HCI are obviously hypsochromic shifted compared with the reported spectra of the complex in neutral media. In the mean time, the thermal stability of the complex is increased. The first order kinetics for the decoloration process of the acidichromic product of the opening form was determined and the lifetimes of these products are 180 and 200 s, respectively.
Similar content being viewed by others
References
Chu, N. Y. C., 4n+2 system: Spirooxazines, inPhotochromism, Molecules and System (eds. Durr, H., Bouas-Laruent, H.), Chapt. 10, Amsterdam: Elsevier, 1990.
Chu, N. Y. C., Photochromism of spiroindolinonaphthooxazine (I): Photophysical properties,Can. J. Chem., 1983, 61: 300.
Schneider, S., Investigation of the photochromic effect of spiroindolinonaphthooxazine derivatives by time-resolved spectroscopy,Phys. Chem., Neue Folge, 1987, 154: 91.
Fan, M. G., Ming, Y. F., Liang, Y. C.et a1., Studies on relations between molecular structure and photwhromic properities of indolinonaphthmxazine,J. Chem. Soc., Perkin Trans. II., 1994, 1397.
Bohne, C., Fan, M. G., Scaiano, J. C.et a1., Laser photolysis studies of photochromic processes of spimxazines: solvent effect on merocyanine behaviour,J. Photochem. Photobiol., A: Chem., 1992, 66: 79.
Rys, P., Weber, R., Wu, Q. L., Light-induced change of the molecular charge in a spironaphthmxazine compound,Can. J. Chenz., 1993, 71: 1828.
Zhang, X. Y., Jin, S., Fan, M. G.et al., The structure character of photoproducts of indolinospiroosazines,Res. Chem. Intermed., 1995, 21: 17.
Lee, Y. S., Kim, J. G. Huh, Y. D.el a1., Thermochromism of spiropyrans and spirmxazines,J. Korean Chem. Soc., 1994, 38(12): 864.
Author information
Authors and Affiliations
Additional information
Project supported by the National Natural Science Foundation of China and the Chinese Academy of Sciences.
Rights and permissions
About this article
Cite this article
Yongchao, L., Ming, Y., Fan, M. et al. Acidichromism of indolinospirooxazines in isopropanol. Sc. China Ser. B-Chem. 40, 535–540 (1997). https://doi.org/10.1007/BF02875424
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02875424