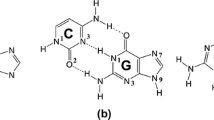
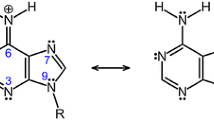
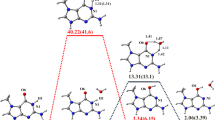
Abstract
All O-alkylated DNA bases and nucleosides possess alkyl groups considerably more labile than those in N-alkylated bases and nucleosides, being prone to degradation through loss of the alkyl group at strongly acidicpH. The strength of the bond between the alkyl group and the atom on the base to which it is bound is calculated here using the semiempirical INDO-SCF-MO method, comparison being made between O6-alkylguanines, O4-alkylthymines and N7-alkylguanines. The results, calculated for many different alkyl groups, predict that the strength of this bond at acidicpH would be appreciably lower for the O-alkylated bases than for the N7-alkylguanines, but that increase ofpH would serve to stabilise this bond for the O-alkylated bases. These predictions are in good accord with experimental findings.
Similar content being viewed by others
References
Abbott P and Saffhill R 1977Nucleic Acid Res. 4 761
Allore B D, Queen A, Blonski W J and Hruska F E 1983Can. J. Chem. 61 2397
Beranek D T, Weis C C and Swenson D H 1980Carcinogenesis 1 595
Bhanot O S and Ray A 1986Proc. Natl. Acad. Sci. USA 83 7348
Duncan R H 1991Indian J. Chem. A30 739
Duncan R H and Davies G S 1989J. Theor. Biol. 140 345
Dunn D B and Hall R H 1975 InHandbook of biochemistry and molecular biology. Nucleic acids 3rd edn. (ed.) G P Fasman (Cleveland, OH: CRC Press) vol 1, pp. 65–215
Ford G P 1986J. Am. Chem. Soc. 108 5104
Ford G P and Scribner J D 1983J. Comput. Chem. 4 594
Ford G P and Scribner J D 1990Chem. Res. Toxicol. 3 219
Frei J V and Lawley P D 1975Chem. Biol. Interact. 10 413
Frei J V, Swenson D H, Warren W and Lawley P D 1978Biochem. J. 174 1031
Gerdil R 1961Acta Crystallogr. 14 333
Kanakavel M, Chandrasekhar J, Subramanian S and Singh S 1976Theor. Chim. Acta 43 185
Kleihues P and Magee P N 1971J. Neurochem. 20 595
Kusmierek J T and Singer B 1976Nucleic Acid Res. 3 989
Lawley P D 1984 InChemical carcinogens 2nd edn (ed.) C E Searle (Washington: American Chemical Society) p. 324
Lawley P D and Thatcher C J 1970Biochem. J. 116 696
Loveless A 1969Nature (London) 223 206
Ludlum D B 1970J. Biol. Chem. 245 477
Magee P N and Farber E 1962Biochem. J. 83 114
Margison G P and Kleihues P 1975Biochem. J. 148 521
Mohammed S N and Hopfinger A J 1980J. Theor. Biol. 87 401
O’Connor P C, Capps M J and Craig A W 1973Br. J. Cancer 27 153
O’Connor P C, Capps M J, Craig A W, Lawley P D and Shah S A 1972Biochem. J. 129 519
Osborne G A 1984 InChemical carcinogens 2nd edn (ed.) C E Searle (Washington: American Chemical Society) p. 485
Pederson L G, Darden T A, Deerfield D W, Anderson M W and Hoel D G 1988Carcinogenesis 9 1553
Pegg A E 1977Adv. Cancer Res. 25 195
Pohorille A and Loew G H 1985Biophys. Chem. 22 45
Pople J A and Beveridge D L 1967Approximate molecular orbital theory (New York: Academic Press) p. 111
Pople J A, Beveridge D L and Dobosh P A 1967J. Chem. Phys. 47 2026
Preston B D, Singer B and Loeb L A 1986Proc. Natl. Acad. Sci. USA 83 8501
Preussmann R and Stewart B W 1984 InChemical carcinogens 2nd edn (ed.) C E Searle (Washington: American Chemical Society) p. 643
Psoda A, Kierkaszuk D, Pohorille A, Geller M, Kusmierek J T and Shugar D 1981Int. J. Quantum Chem. 20 543
Richardson F C, Dyroff M C, Boucheron J A and Swenberg J A 1983Carcinogenesis 6 625
Schoental R 1969Biochem. J. 114 559
Singer B 1975Prog. Nucleic Acid Res. Mol. Biol. 15 219
Singer B 1976Nature (London) 264 333
Singer B 1986Cancer Res. 46 4879
Singer B, Kroger M and Carrano M 1978Proc. Natl. Acad. Sci. USA 75 1722
Swann P F and Magee P N 1971Biochem. J. 125 841
Thewalt U, Bugg C E and Marsh R E 1971Acta Crystallogr. B27 2358
Wiberg K A 1968Tetrahedron 24 1083
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lyngdoh, R.H.D. Comparison of alkyl group labilities in O-and N-alkylated DNA bases: A semiempirical molecular orbital study. Proc. Indian Acad. Sci. (Chem. Sci.) 105, 253–263 (1993). https://doi.org/10.1007/BF02866914
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02866914