Abstract
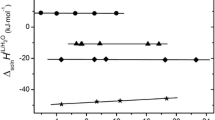
The solvation behaviour of the salts, silver bromate, silver acetate and silver sulphate has been investigated in the binary mixtures of N,N-dimethyl formamide (dmf) and water. The Gibbs energies of transfer of these salts from water to water-dmf mixtures have been calculated from the solubilities of the salts in these mixtures. The transfer free energies of the salts were split into their ionic contributions by using the transfer-free energy of the silver ion in these mixtures obtained on the basis of negligible liquid junction potential (nLjp) method from independentemf measurements. The solvent transference number, Δ ofdmf, was determined byemf measurements using a suitable galvanic cell with transference. The results have been interpreted in terms of a heteroselective solvation of the salts with the silver ion selectively solvated bydmf and the anions by water.
Similar content being viewed by others
References
Alexander R, Parker A J, Sharp J H and Waghorne W E 1972J. Am. Chem. Soc. 94 1148
Barraque C, Vedel J and Trémillon B 1968Bull. Chem. Soc. France 8 3421
Carmody W 1928J. Am. Chem. Soc. 51 2901
Faulkner L R and Bard A J 1968J. Am. Chem. Soc. 51 2901
Geller B E 1961Russ. J. Phys. Chem. 35 1090
Giridhar V V and Kalidas C 1982J. Sol. Chem. 11 539
Giridhar V V and Kalidas C 1983Indian J. Chem. A22 224
Ivanova T M and Geller B E 1961Zh. Fiz. Khim. 35 1221
Janardhanan S and Kalidas C 1980Bull. Chem. Soc. Jpn 53 2363
Janardhanan S and Kalidas C 1982Monatsh. Chem. 113 691
Kalidas C and Sivaprasad P 1979Indian J. Chem. A17 79
Kundu K K and Parker A J 1981J. Sol. Chem. 10 847
Schneider H 1976aElectrochim. Acta 21 711
Schneider 1976bTopics in Curr. Chem. 68 105
Singh D, Lal Bahadur and Ramana Murti M V 1977J. Sol. Chem. 6 703
Stroka J and Schneider H 1980Polish J. Chem. 54 1805
Subramanian S, Rao S C A V S S and Kalidas C 1981Indian J. Chem. A20 723
Vierk A L 1950Z. Anorg. Allg. Chem. 261 238
Wagner C 1966Advances in electrochemistry and electrochemical engineering, 4th edn. (New York: Wiley Interscience) 1–46
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Giridhar, V.V., Kalidas, C. Ion-solvent interactions of some silver salts in N,N-dimethyl formamide-water mixtures at 30°C. Proc. Indian Acad. Sci. (Chem. Sci.) 93, 795–800 (1984). https://doi.org/10.1007/BF02866340
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02866340