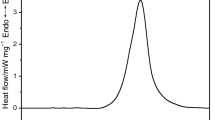
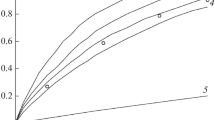
Abstract
Thermal decomposition characteristics of 2,4 dinitroanilino acetic acid, 2,4,6 trinitro anilino acetic acid and their methyl and ethyl esters have been investigated by DTA. It is concluded from a study of the kinetic parameters of their decomposition, intramolecular hydrogen bonds existing in these compounds and mass spectral fragmentation characteristics of ethyl esters that these compounds decompose by the loss of OH through a cyclic intermediate formed by the intramolecular hydrogen bonding between the amino hydrogen atom and ortho nitro group.
Similar content being viewed by others
References
Abderhalden E and Blumberg P 1910J. Chem. Soc. 98 I 371
Bellamy L J, Hallam H E and William R L 1958Trans. Faraday. Soc. 54 1120
Fedoroff B T (ed.) 1960Encyclopedia of explosives and related items (New Jersey: Picatinny arsenal, Dover) Vol. 1, p.A420
Fields E K and Meyerson S 1968J. Org. Chem. 33 4487
Fields E K and Meyerson S 1975 inAdvances in free radical chemistry (ed.) G H Williams (London: Elek Science) Vol. 5, p. 101
Hellberg L H, Prodanovich M J and Stults F 1972J. Heterocycl. Chem. 9 401
Kissinger H E 1957Anal. Chem. 29 1702
Kwart H and Taagepara M R 1964Tetrahedron Lett. 18 1099
Maksimov Yu Ya 1969Zh. Fiz. Khim. 43 725 (Chem. Abstr. 196971 49032 s)
Maksimov Yu Ya 1971Zh. Fiz. Khim. 45 793 (Chem. Abstr. 197175 34938 a)
Maksimov Yu Ya and Kogut E N 1978Zh. Fiz. Khim. 52 1400 (Chem. Abstr. 197889 107565 c)
Matveev V G, Dubikhin V V and Nazin G M 1976Kinet. Catal. 17 280 andChem. Abstr. 197685 93554 b
Matveev V G, Dubikhin V V and Nazin G M 1978aIzv. Akad. Nauk. SSSR, Ser. Khim. 783 andChem. Abstr. 197889 23513 w
Matveev V G, Dubikhin V V and Nazin G M 1978bIzv. Akad. Nauk. SSSR, Ser Khim. 474 andChem. Abstr. 197888 151 803 g
Musso R C 1967 quoted by Meyerson S, Vender Haar R W and Fields E K 1972J. Org. Chem. 37 4114
Ozawa T 1965Bull. Chem. Soc. Jpn. 38 1881
Rao K U B 1982Studies on nitro anilino acetic acids Ph.D. thesis, University of Poona, Pune
Roberts J D and Caeserio M L 1965Principles of organic chemistry (New York: Benjamin Inc.) p. 77
Studier M H, Moore L P, Hayat R and Matsuoka S 1970Biochem. Biophys. Res. Commun. 40 894
Torssel K and Ryhage R 1965 quoted by Meyerson S, Vender Haar R W and Fields E K 1972J. Org. Chem. 37 4114
Zeman S 1980Thermochim. Acta 39 117
Author information
Authors and Affiliations
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/BF02840460.
Rights and permissions
About this article
Cite this article
Rao, K.U.B., Yoganarasimhan, S.R. Thermal decomposition of some nitroanilinoacetic acids. Proc. Indian Acad. Sci. (Chem. Sci.) 95, 309–314 (1985). https://doi.org/10.1007/BF02864195
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02864195