Abstract
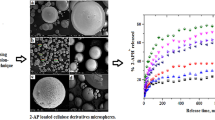
Indomethacin was microencapsulated with ethylcellulose using a modified spherical agglomeration process, aiming at a sustained release preparation without side effects on the stomach. The surface morphology of the microcapsules was examined using scanning electron microscopy. The microcapsules were porous and spherical, and their porosity increased with increasing the viscosity of ethylcellulose.In vitro dissolution process followed Higuchi’s diffusion model for first 3 hr. Release rate of the drug from microcapsules decreased as the viscosity of ethylcellulose or the weight ratio of indomethacin to ethylcellulose was decreased. The release rate also decreased with increasing the microcapsule size. The microcapsules induced less gastric ulcer in rats than raw drug.
Similar content being viewed by others
Literature Cited
O’Brien, W.M.: Indomethacin: a survey of clinical trials.Clin. Pharmacol. Ther. 9, 94(1968).
Smyth, C.J. and Percy, J.S.: Comparison of indomethacin and phenylbutazone in acute gout.Ann. Rheum. Dis. 32: 351(1973).
Kwan, K.C., Breault, G.O., Unbenhauer, E.R., McMahon, F.G. and Duggan, D.E.: The kinetics of indomethacin absorption, elimination and enterohepatic circulation in man.J. Pharmacokinet. Biopharm. 4, 225(1976).
Gillman, A.G., Goodman, L.S. and Gillman, A.:Goodman and Gillman’s The pharmacolgoical basis of therapeutics. 6th ed., Macmillan, 705(1980).
Vane, J.R.: Inhibition of prostaglandin synthesis as a mechanism for aspirin-like drugs.Nature 231, 232(1971).
Vidras, N.J., Reid, M.J., Bohidar, N.R. and Plakogiannis, F.M.: Medicament release from suppository bases I: Physicochemiacl characteristics and bioavailability of indomethacin in rabbits.J. Pharm. Sci. 71, 945(1982).
Nitto Electric Industrial Co., Ltd.:Jpn. Kokai Tokkyo Koho 81, 40, 608(1981).
Alvan, C., Orme, M., Bertilesson, L., Ekstrand, R. and Palmer, L.: Pharmackinetics of indomethacin.Clin. Pharmacol. Ther. 18, 368(1974).
Shima, K., Matsusaka, C., Hirose, M., Noguchi, T. and Yamahira, Y.: Biopharmaceutical characteristics of indomethacin gel ointment.Chem. Pharm. Bull. 29, 2338 (1981).
Ishiguro, F., Minamiki, A., Kanagawa, T., Kawajiri, A., Ishiguro, J. and Koizumi, T.: Percutaneous absorption of indomethacin from plasters.Yakuzaigaku 42, 324(1928).
Nitto Electric Industrial Co., Ltd.:Jpn. Kokai Tokkyo Koho 81, 51, 412(1981).
Rowe, J.S., and Carless, J.E.: Encapsulation of indomethacin.UK Patent GB 2075458 Nov. 18, (1981).
Dempski, R.E., Mehta, G.N. and Saboe, J.S.: Sustained release indomethacin.U.S. Patent 4173626 Nov. 6, (1979).
Takeda, Y., Nambu, N. and Nagai, T.: Microencapsulation and bioavailability in beagle dogs of indomethacin.Chem. Pharm. Bull. 29, 264 (1981).
Rowe, J.S. and Carless, J.E.: Comparison of thein vitro dissolution behavior of various indomethacin formulations with theirin vivo bioavailability.J. Pharm. Pharmacol. 33, 561(1981).
Mizushima, Y., Wada, Y., Etoh, Y. and Watanabe, K.: Anti-inflammatory effects of indomethacin ester incorporated in a lipid microsphere.J. Pharm. Pharmacol. 35, 398(1983).
Eckenhoff, B., Theeuwes, F. and Urquhart, J.: Osmotically actuated dosage forms for rate controlled drug delivery.Pharmaceutical Technology Jan, 35(1981).
Bertouch, J.V., Harrington, B. and Brooks, P.M.: Pharmacokinetics of an osmotically controlled delivery indomethacin preparation in normal volunteers.Clin. Exp. Pharmacol. 10, 411(1983).
Kawashima, Y., Ohno, H. and Takenaka, H., Preparation of spherical matrices of prolonged release drugs from liquid suspension.J. Pharm. Sci. 70, 913(1981).
Satoh, H., Guth, P.H. and Grossman, M.I.: Role of food in gastrointestinal ulceration produced by indomethacin in the rat.Gastroenterology 83, 210(1982).
Kawashima, Y. and Capes, C.E.: Further studies of spherical agglomeration in a stirred vessel.Powder Technology 13, 279(1976).
Deasy, P.B., Brophy, M.R., Ecanow, B. and Joy, M.: Effect of ethylcellulose grade and sealant treatments on the production andin vitro release of microencapsulated sodium salicylated.J. Pharm. Pharmacol. 32, 15(1980).
Author information
Authors and Affiliations
Additional information
A part of this work is taken from a dissertation submitted by H.J. Lee to the Graduate School, College of Pharmacy, Seoul National University, Seoul, Korea, in partial fulfillment of the requirements for the degree of Master of Science.
Rights and permissions
About this article
Cite this article
Lee, HJ., Lee, MH. & Shim, CK. Preparation and evaluation of ethylcellulose microcapsules of indomethacin. Arch. Pharm. Res. 7, 33–40 (1984). https://doi.org/10.1007/BF02856919
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02856919