Abstract
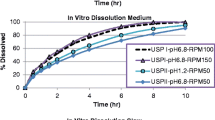
Many mathematical models have been proposed for establishing an in vitro/in vivo correlation (IVIVC). The traditional IVIVC model building process consists of 5 steps: deconvolution, model fitting, convolution, prediction error evaluation, and cross-validation. This is a time-consuming process and typically a few models at most are tested for any given data set. The objectives of this work were to (1) propose a statistical tool to screen models for further development of an IVIVC, (2) evaluate the performance of each model under different circumstances, and (3) investigate the effectiveness of common statistical model selection criteria for choosing IVIVC models. A computer program was developed to explore which model(s) would be most likely to work well with a random variation from the original for-mulation. The process used Monte Carlo simulation techniques to build IVIVC models. Data-based model selection criteria (Akaike Information Criteria [AIC],R 2) and the probability of passing the Food and Drug Administration “prediction error” requirement was calculated. To illustrate this approach, several real data sets representing a broad range of release profiles are used to illustrate the process and to demonstrate the advantages of this automated process over the traditional approach. The Hixson-Crowell and Weibull models were often preferred over the linear. When evaluating whether a Level A IVIVC model was possible, the model selection criteria AIC generally selected the best model. We believe that the approach we proposed may be a rapid tool to determine which IVIVC model (if any) is the most applicable.
Similar content being viewed by others
References
McClelland GA, Sutton SC, Engle K, Zentner GM. The solubility modulated osmotic pump: in vitro/in vivo release of diltiazem hydrochloride.Pharm Res. 1991;8:88–92.
Sunesen VH, Pedersen BL, Kristensen HG, Mullertz A. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media.Eur J Pharm Sci. 2005;24:305–313.
Cheung RY, Kuba R, Rauth AM, Wu XY. A new approach to the in vivo and in vitro investigation of drug release from locoregionally delivered microspheres.J Control Release. 2004;100:121–133.
Liu Y, Schwartz JB, Schnaare RL, Sugita ET. A multi-mechanistic drug release approach in a bead dosage form and in vitro/in vivo correlations.Pharm Dev Technol. 2003;8:409–417.
FDA. FDA Guidance for Industry. Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Containing Certain Active Moieties/Active Ingredients Based on a Biopharmaceutics Classification System. Rockville, MDFDA. 2000, Available at: http://www.fda.gov/cder/guidance/index/htm. Accessed April 11, 2005.
Gillespie W. Convolution-based approaches for in vitro in vivo correlation modeling. In: Young D, Devane J, Butler J, eds.In Vitro in Vivo Correlations. Vol. 423. New York, NY: Plenum Press; 1997:53–65.
Pitsin M, Sathyan G, Gupta S, Verotta D. A semiparametric deconvolution model to establish in vivo-in vitro correlation applied to OROS oxybutynin.J Pharm Sci. 2001;90:702–712.
Buchwald P. Direct, differential-equation-based in-vitro-in-vivo correlation (IVIVC) method.J Pharm Pharmacol. 2003;55:495–504.
Gillespie WR. Convolution-based approaches for in vivo-in vitro correlation modeling.Adv Exp Med Biol. 1997;423:53–65.
Veng-Pedersen P, Gobburn JV, Meyer MC, Straughn AB. Carbamazepine level-A in vivo-in vitro correlation (IVIVC): a scaled convolution based predictive approach.Biopharm Drug Dispos. 2000;21:1–6.
Mendell-Harary J, Dowell J, Bigora S. Nonlinear in vitro-in vivo correlations In: Young D, Devane J, Butler J, eds.Vol 423.In Vitro-In Vivo Correlations. New York, NY: Plenum Press; 1997:199–206.
Thombre AG, Cardinal JR, DeNoto AR, Herbig SM, Smith KL. Asymmetric membrane capsules for osmotic drug delivery. I. Development of a manufacturing process.J Control Release. 1999;57:55–64.
Mano T, Stevens RW, Ando K, et al. Novel imidazole compounds as a new series of potent, orally active inhibitors of 5-lipoxygenase.Bioorg Med Chem. 2003;11:3879–3887.
Marquardt D. An algorithm for least-squares estimation of nonlinear parameters.SIAM J Appl Math. 1963;11:431–441.
Tzeng TB, Fung HL. Pharmacodynamic modelling of the in vitro vasodilating effects of organic mononitrates.J Pharmacokinet Biopharm. 1992;20:227–251.
Akaike H.Information theory and the extension of the maximum likelihood principle. Proceedings of the Second International Symposium on Information Theory, 1973; Budapest Akailseoniai-Kiudo; Budapest: Akailseoniai-Kiudo; 1973:267–281.
Kullback S.Information Theory and Statistics. New York, NY: Dover Publications; 1968.
Hurvich C, Tsai C-L. Regression and Time Series Model Selection in Small Samples.Biometrika. 1989;76:297–307.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: May 5, 2006
Rights and permissions
About this article
Cite this article
Sutton, S.C., Hu, M. An automated process for building reliable and optimal in vitro/in vivo correlation models based on Monte Carlo simulations. AAPS J 8, 35 (2006). https://doi.org/10.1007/BF02854901
Received:
Accepted:
DOI: https://doi.org/10.1007/BF02854901