Abstract
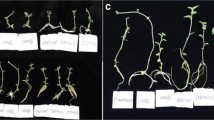
Potato (Solanum tuberosum L.) cvs. AC Brador and Shepody grownin vitro exhibit plantlets with a short, rosette-type growth habit, and restricts multi plication rates. In contrast, Russet Burbank develops into plantlets with adequate internode lengthin vitro. Cool-white fluorescent lamps provided irradiation for wooden boxes fitted with yellow plexiglass filters. The resultant irradiation lacked wavelengths from 380 to 525 nm. A box fitted with clear plexiglass was the control. Internode length of plantlets was ca.1.0 cm for all three cultivars when grown in boxes with yellow filters, and was 0.5 cm when clear filters were used. The number of nodes, haulm area, stem length, haulm fresh and dry weight of cvs. AC Brador and Shepody were significantly greater in boxes with yellow filters. Potato cvs. AC Belmont, Eramosa, Hunter, Huron, Mirton Pearl, Raritan and York all have a short growth habitin vitro and stem length was increased by the use of a yellow filter. The incidence of intumescences or oedemas on leaves of potato plantletsin vitro was reduced from 60 to seven percent for AC Brador and from 70 to 10% for Shepody with the use of yellow filters. Blue-green light is therefore putatively involved in the occurrence of intumescences on potato tissuesin vitro.
Similar content being viewed by others
Literature Cited
Ahmad, M. and A.R. Cashmore. 1993.HY4 gene ofA. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:162–166.
Ahuja, M.R. 1965. Genetic control of tumour formation in higher plants. Quart Rev Biol 40:329–340.
Bennett, J.H., E.H. Lee, D.T. Krizek, R.A. Olsen, and J.C. Brown. 1982. Photochemical reduction of iron. II. Plant related factors. J Plant Nutrition 5:335–344.
Britz, S.J. and J.C. Sager. 1990. Photomorphogenesis and photoassimilation in soybean and sorghum under broad spectrum of bluedeficient light sources. Plant Physiol 94:448–454.
Chailakhyan, M.Kh., A.V. Makeev, N.P. Aksenova, T.N. Konstantinova, and A.T. Mokronosov. 1992. Effects of daylength and light spectral composition on morphogenesis and photosynthesis of plants of the potatoSolatium andigenum (Juz.et Buk.). Soviet Plant Physiol 39:129–134.
Chee, R. and R.M. Pool. 1989. Morphogenic responses to propagule trimming, spectral irradiance, and photoperiod of grapevine shoots reculturedin vitro. J Amer Soc Hort Sci 114:350–354.
Cosgrove, D.J. 1981. Rapid suppression of growth by blue light. Plant Physiol 67:584–590.
Desjardins, Y. 1995. Photosynthesisin vitro — on the factors regulating CO2 assimilation in micropropagation systems. Acta Hortic 393:45–57.
Douglas, G.E. 1907. The formation of intumescences on potato plants. Bot Gaz 43:233–250.
George, E.F. 1993. Plant Propagation Through Tissue Culture. Exegenics Ltd., England. 574 pp.
Hangarter, R.P. and T.C. Stasinopoulos. 1991. Repression of plant tissue culture growth by light is caused by photochemical change in the culture medium. Plant Sci 79: 253–257.
Lang, S.P. and T.W. Tibbitts. 1983. Factors controlling intumescence development on tomato plants. J Amer Soc Hort Sci 108:93–98.
Lledó, M.D., M.B. Crespo, and J.B. Amo-Marco. 1994. Ethylene production and lenticel hypertrophyin vitro cultures ofPopulus euphratica Oliver. Proc. VIII Intl. Cong. Plant Tiss. & Cell Cult., Florence, Italy, 12–17 June, 1994. Abstr. # S14-26:218.
McLachlan, J. and R.G.S. Bidwell. 1983. Effects of colored light on the growth and metabolismof Fucus embryos and apices in culture. Can J Bot 61:1993–2003.
Métraux, J.-P. 1987. Gibberellins and plant cell elongation. In: Plant Hormones and Their Role in Plant Growth and Development. Chap. E-3. [Ed: P.J. Davies]. Martinus Nijhoff, Dordrecht: 296–317.
Morrow, R.C. and T.W. Tibbitts. 1988. Evidence for involvement of phytochrome in tumor development in plants. Plant Physiol 88:1110–1114.
Petitte, J. M. and D.P. Ormrod. 1986. Factors affecting intumescence development on potato leaves. HortScience 21:493–495.
Rangarajan, A. and T.W. Tibbitts. 1994. Exposure with far-red radiation for control of oedema injury on “Yale” ivy geranium. HortScience 29:38–40.
Sagi, A. and I. Rylski. 1978. Differences in susceptibility to oedema in two tomato cultivais growing under various light intensities. Phytoparasitica 6:151–153.
Seabrook, J.E.A. 1987. Changing the growth and morphology of potato plantletsin vitro by varying the illumination source. Acta Hortic 212:401–410.
Seabrook, J.E.A., and L.K. Douglass. 1994. Reduction in vigour of leafless potato cuttingsin vitro. Potato Res 37:365–371.
Senger, H., and E.D. Iipson 1987. Problems and prospects of blue and ultraviolet light effects. In: Phytochrome and Photoregulation in Plants. (Ed: M. Furuya). Academic Press, Tokyo: 315–331.
Sokal, R.R., and F.J. Rohlf. 1987. Introduction to Biostatistics. 2nd Edn. W.H. Freeman & Co., New York. 363 pp.
Wilson, DA, R.C. Weigel, R.M. Wheeler, and J.C. Sager. 1993. Light spectral quality effects on the growth of potato (Solanun tuberosum L.) nodal cuttingsin vitro. In Vitro Cell Dev Biol 29:5–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seabrook, J.E.A., Douglass, L.K. Prevention of stem growth inhibition and alleviation of intumescence formation in potato plantletsin vitro by yellow filters. Am. J. Pot Res 75, 219–224 (1998). https://doi.org/10.1007/BF02854216
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02854216