Abstract
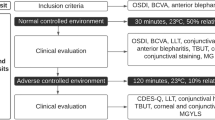
The tolerability of brimonidine tartrate 0.15%—referred to as bromonidine-Purite® 0.15% in this study—was compared with brimonidine tartrate 0.2% in irritated eyes of healthy volunteers as well as patients with glaucoma or ocular hypertension (N=20) in a 2-week, single-center, randomized, double-masked, crossover study. Participants were evaluated at days 0, 7, 11, and 15. At day 0, they were exposed to a controlled adverse environment (CAE), in which humidity, temperature, and airflow was regulated, for up to 90 minutes. Participants who reported a sufficient level of bilateral ocular discomfort during exposure to the CAE were enrolled in this study and received 1 drop of brimonidine-Purite 0.15% in 1 eye and 1 drop of brimonidine tartrate 0.2% in the contralateral eye. Immediately following instillation, participants were asked to indicate their preference for either study medication. The study medications were not used between days 0 and 7. From day 7 to 10, participants administered either drug bilaterally. On day 11, the treatment was crossed over, and participants were asked to compare the preferred medication with their previous regimen. They continued to administer the drug bilaterally twice daily until day 15, when the preferred medication was again compared with the previous regimen. Following CAE exposure at visit 1, 70.6% of the participants preferred brimonidine-Purite 0.15% over brimonidine tartrate 0.2% and indicated that it was significantly more comfortable than brimonidine tartrate 0.2% (P=.009). When given brimonidine-Purite 0.15% first before switching to brimonidine tartrate 0.2%, 80% of participants preferred brimonidine-Purite 0.15% (P=.012). When given brimonidine tartrate 0.2% first before switching to brimonidine-Purite 0.15%, 85% preferred brimonidine-Purite 0.15% (P=.001). The results of this study suggest that brimonidine-Purite 0.15% is significantly more comfortable than brimonidine tartrate 0.2% in patients with irritated eyes.
Similar content being viewed by others
References
Melamed A, David R. Ongoing clinical assessment of the safety profile and efficacy of brimonidine compared to timolol: year-three results.Clin Ther. 2000;22:103–111.
DuBiner HB, Mroz M, Shapiro AM, Dirks MS. A comparison of the efficacy and tolerability of brimonidine and latanoprost in adults with open-angle glaucoma or ocular hypertension: a three-month, multicenter, randomized, double-masked, parallel-group trial.Clin Ther. 2001;23:1969–1983.
Lee DA, Gornbein J, Abrams C. The effectiveness and safety of brimonidine as mono-, combination, or replacement therapy for patients with primary open-angle glaucoma or ocular hypertension: a post hoc analysis of an open-label community trial.J Ocul Pharmacother. 2000;16:3–18.
Lee DA. Efficacy of brimonidine as replacement therapy in patients with open-angle glaucoma or ocular hypertension.Clin Ther. 2000;22:53–65.
Alphagan® (brimonidine tartrate ophthalmic solution 0.2%) prescribing information. Allergan, Inc., Irvine, Calif.
De Saint Jean M, Debbasch C, Brignole F, Rat P, Warnet JM, Baudouin C. Toxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cells.Curr Eye Res. 2000;20:85–94.
Rozen S, Abelson M, Giovanoni A, Welch D. Assessment of the comfort and tolerance of 0.5% carboxymethylcellulose preserved with Purite (Referesh Tears) in dry eye sufferers [abstract].Invest Ophthalmol Vis Sci. 1998;39:B343.
Katz LJ. Twelve-month evaluation of brimonidine-purite versus brimonidine in patients with glaucoma or ocular hypertension.J Glaucoma. 2002;11:119–126.
Ousler GW, Gomes PJ, Crampton HJ, Abelson MB. The effects of a lubricant eye drop on the signs and symptoms of computer vision syndrome (CVS) exacerbated in a controlled adverse environment [abstract].Invest Ophthalmol Vis Sci. 1999;40:S540.
Ousler GW, Crampton HJ, Abelson MB. An evaluation of signs and symptoms associated with caruncle and pila semi-lunaris after exposure to a controlled adverse environment [abstract].Invest Ophthalmol Vis Sci. 2000;41:S928.
Ousler GW, Abelson MB, Nally LA, Welch D. An evaluation of the time-to ‘natural compensation’ or improved ocular discomfort during exposure to a controlled adverse environment (CAE) in normal and dry eye populations [abstract].Cornea. 2000;19(suppl):2467.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mundorf, T., Wilcox, K.A., Ousler, G.W. et al. Evaluation of the comfort of Alphagan® P compared with Alphagan® in irritated eyes. Adv Therapy 20, 329–336 (2003). https://doi.org/10.1007/BF02849799
Issue Date:
DOI: https://doi.org/10.1007/BF02849799