Abstract
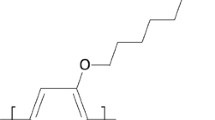
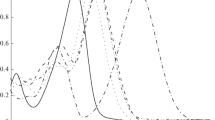
Photochemical reactions of poly(3-butoxythiophene-2,5-diyl) with chloroform under irradiation with light were studied. The reactions were separately carried out under air, oxygen, and nitrogen. The obtained results showed that this reaction belongs to the pseudo-first-order reaction with a rate constantk obs of 1.4×10−5 s−1 at room temperature. The presence or absence of air, oxygen, and nitrogen did not have obvious effects on the reaction rate under irradiation with light.
Similar content being viewed by others
References
Chen, S.A., Hua, M.Y., 1993. Structure and doping level of the self-acid-doped conjugated conducting polymers: Poly[n-(3′-thienyl)alkanesulfonic acids.Macromolecules,26:7108–7110.
Friend, R.H., Gymer, R.W., Holmes, A., Burroughes, J.H., Marks, R.N., Taliani, C., Bradley, D.D.C., Dos Santos, D.A., Bredas, J.L., Logdlund, M.,et al., 1999. Electroluminescence in conjugated polymers.Nature (London),397:121–128.
Heeger, A., 2001. Semiconducting and metallic molymers: Mhe fourth generation of polymeric materials.Angew. Chem. Int. Ed.,40:2591–2611.
Ikenoue, Y., Saida, Y., Kira M., Tomozawa, H., Yashima, H., Kobayashi, M., 1990. A facile preparation of a self-doped conducting polymer.J. Chem. Soc. Chem. Commun.,23:1694–1695.
Ikenoue, Y., Tomozawa, H., Saida Y., Kira, M., Yashima, H., Kobayashi, M., 1991. Evaluation of electrochromic fast-switching behavior of self-doped conducting polymet.Synth. Met.,40:333–340.
Kong, X.X., Kulkarni, A.P., Jenekhe, S.A., 2003. Phenothiazine-based conjugated polymers: Synthesis, electrochemistry, and light-emitting properties.Macromolecules,36:8992–8999.
Miyazaki, Y., Kanbara, T., Osakada, K., Yamamoto, T., 1993. Preparation of poly(alkoxythiophene-2,5-diyl)s by organometallic process and doping-undoping behaviors of the polymers.Chem. Lett.,22:415–418.
Petrushenko, K.B., Kylba, L.V., Smirnow, V.I., Shevchenko, S.G., 2001. Electron transfer in the photochemical reactions of phenothiazine with halomethanes.Russ. Chem. Bull. Int. Ed.,50:798–804.
Shimamori, H., Hanamura, K.I., Tatsumi, Y., 1993. Rates and efficiencies of contact-ion-pair formation in photolyzed mixtures of TMPD with halogenated compounds in nonpolar solvents.J. Phys. Chem.,97:3545.
Yamamoto, T., 2002. π-conjugated polymers with electronic and optical functionalities: Preparation by or ganometallic polycondensation, properties, and applications.Macromol Rapid Commun.,23:583–606.
Yamamoto, T., Omote, M., Miyazaki, Y., Kashiwazaki, A., Lee, B.L., Kanbara, T., Osakada, K., Inoue, T., Kubota, K., 1997. Poly(thiophene-2,5-diyl)s with a crown ethereal subunit, preparation, optical properties, and n-doping state stabilized against air.Macromolecules,30: 7158–7165.
Yamamoto, T., Sakamaki, M., Fukumoto, H., 2003. π-doping behavior of water-soluble π-conjugated poly[3-(3-sulfopropyl)thiophene]: Kinetic and spectroscopic studies.Synth. Met.,139:169–173.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mokhtar, I., Takakazu, Y. & Patigul, I. Photochemical reactions of poly(3-butoxythiophene-2,5-diyl) with chloroform. J. Zheijang Univ.-Sci. B 6, 722–724 (2005). https://doi.org/10.1007/BF02842429
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02842429