Abstract
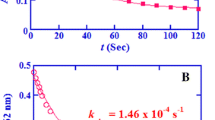
The kinetics of oxidation of a typical dipeptide glycylglycine (GG) by bromamine-T have been studied in HClO4 medium at 40°C. The rate shows first-order dependence on [BAT]0 and is fractional order in [GG]0 which becomes independent of [substrate]0 at higher [GG]0. At [H+ ] > 0.02mol dm−3, the rate is inverse fractional in [H+ ] but is zero order at lower [H+ ] (≤0.02 mol dm−3). Variation in ionic strength or dielectric constant of the medium had no significant effect on the rate. The solvent-isotope effect was measured and\(k'_{H_2 O} /k'_{H_2 O} \)= 1.45. Proton inventory studies have been made. The reaction has been studied at different temperatures (308-323 K) and activation parameters have been computed.
Similar content being viewed by others
References
Akabori S, Narita K, Toki K and Hanafusa H 1954J. Chem. Soc. Jpn., Pure Chem. Sec. 75 782
Amis E S 1966Solvent effects in reaction rates and mechanisms (New York: Academic Press)
Bishop E and Jennings V J 1958Talanta 1 197
Collins C J and Bowman N S 1970Isotope effects in chemical reactions (New York: Van Nostrand) p. 267
Feigl F 1956Spot tests in organic analysis (Amsterdam: Elsevier) p. 341
Fieser L F and Fieser M 1958Organic chemistry (Boston: D C Heath) p. 426
Hammel E F Jr and Glasstone S 1954J. Am. Chem. Soc. 76 3741
Haurowitz F 1950Chemistry and biology of proteins (New York: Academic Press)
Issacs N S 1987Physical organic chemistry (Belfast: Longmans) p. 275
Laidler K J 1973Chemical kinetics (New Delhi: Tata-McGraw Hill) p. 227
Mahadevappa D S, Ananda S, Murthy A S A and Rangappa K S 1983Reaction Kinet. Catal. Lett. 23 181
Mahadevappa D S, Ananda S, Murthy A S A and Rangappa K S 1984Indian J. Chem. A23 17
Mahadevappa D S and Gowda N M M 1975Talanta 22 771
Martin R J L 1955Nature (London) 771
Nair C G R and Indrasenan P 1976Talanta 23 239
Rangappa K S, Mahadevappa D S, Gowda B T and Gowda N M M 1981Microchem. J. 26 375
Synge R L M 1945Biochem. J. 39 351
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iyengar, T.A., Mahadevappa, D.S. Kinetics of oxidation of glycylglycine by bromamine-T in acid medium. Proc. Indian Acad. Sci. (Chem. Sci.) 105, 63–70 (1993). https://doi.org/10.1007/BF02841351
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02841351