Abstract
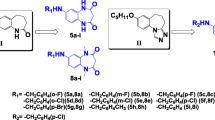
Objective: A series of 4-aryl substituted semicarbazones of levulinic acid (4-oxo pentanoic acid) was designed and synthesized to meet the structural requirements essential for anticonvulsant activity. Methods: All the compounds were evaluated for anticonvulsant activity. Anticonvulsant activity was determined after intraperitoneal (i.p.) administration to mice by maximal electroshock (MES) and subcutaneous metrazol (ScMet) induced seizure methods and minimal motor impairment was determined by rotorod test. Results: A majority of the compounds exhibited significant anticonvulsant activity after intraperitoneal administration. In the present study 4-(4′-fluoro phenyl) levulinic acid semicarbazone emerged as the most active molecule, showing broad spectrum of activity with low neurotoxicity. Unsubstituted levulinic acid semicarbazone was found to be inactive in all the screens. Conclusion: The results obtained validate the hypothesis that presence of an aryl group near the semicarbazone moiety is essential for anticonvulsant activity. The results also indicate that the hydrophilic-hydrophobic site can accommodate hydrophilic groups.
Similar content being viewed by others
References
Aggarwal, N., Mishra, P., 2004. Synthesis of 4-aryl substituted semicarbazones of some terpenes as novel anticonvulsants.J. Pharm. Pharmaceut. Sci.,7(2):260–264.
Camerman, A., Camerman, N., 1980. Stereochemical Similarities in Chemically Different Antiepileptic Drugs.In: Glaser, G.H., Penry, J.K., Woodbury, D.M. (Eds.), Antiepileptic Drugs: Mechanism of Action, Raven Press, New York, p.223–231.
Craig, C.R., 1997. Anticonvulsant Drugs.In: Craig, C.R., Stitzel, R.E. (Eds.), Modern Pharmacology with Clinical Application. 5th Edition, Little Brown and Company, New York, p.391–405.
Dimmock, J.R., Baker, G.B., 1994. Anticonvulsant activities of 4-bromobenzaldehyde semicarbazone.Epilepsia,35(3):648–655.
Edafiogho, I.O., Scott, K.R., 1996. Anticonvulsants.In: Wolff, M.E. (Ed.), Burger's Medicinal Chemistry and Drug Discovery. 5th Edition, Vol. 3, John Wiley and Sons, New York, p. 175–260.
Ho, B., Crider, A.M., Stables, J.P., 2001. Synthesis and structure activity relationships of potential anticonvulsants based on 2-piperidine carboxylic acid and related pharmacophores.Eur. J. Med. Chem.,36:265–286.
Krall, R.L., Penry, J.K., White, B.G., Kupferberg, H.J., Swinyard, E.A., 1978. Anticonvulsant drug development: Anticonvulsant drug screening.Epilepsia,19:409–428.
Malawska, B. 2003. Application of pharmacophore models for the design and synthesis of new anticonvulsant drugs.Mini Rev. Med. Chem.,3:159–165.
McNamara, J.O., 2001. Drugs Effective in the Therapy of the Epilepsies.In: Hardman, J.G., Limbird, L.E., Gilman, A.G. (Eds.), Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10th Edition, The McGraw-Hill, New York, p. 521–547.
Pandeya, S.N., Raja, A.S., 2002. Synthesis of isatin semicarbazones as novel anticonvulsants-role of hydrogen bonding.J. Pharm. Pharmaceut. Sci.,5(3):266–271.
Pandeya, S.N., Aggarwal, N., Jain, J.S., 1999. Evaluation of semicarbazones for anticonvulsant and sedative hypnotic properties.Pharmazie,54:300–302.
Pandeya, S.N., Mishra, V., Ponnilavarsan, I., Stables, J.P., 2000. Anticonvulsant activity ofp-chloro phenyl substituted aryl semicarbazones-the role of primary terminal amino group.Pol. J. pharmacol.,52:283–290.
Porter, R.J., Hessie, B.J., Cereghino, J.J., Gladding, G.D., Kupferberg, H.J., Scoville, B., White, B.G., 1985. Advances in the clinical development of antiepileptic drugs.Fed. Proc.,44(10):2645–2649.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navneet, A., Pradeep, M. Synthesis and evaluation of 4-substituted semicarbazones of levulinic acid for anticonvulsant activity. J. Zheijang Univ.-Sci. B 6, 617–621 (2005). https://doi.org/10.1007/BF02841026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02841026