Abstract
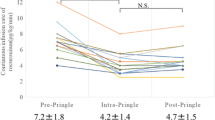
Objective: To compare the dose requirements of continuous infusion of rocuronium and atracurium throughout orthotopic liver transplantation (OLT) in humans. Methods: Twenty male patients undergoing liver transplantation were randomly assigned to two comparable groups of 10 patients each to receive a continuous infusion of rocuronium or atracurium under intravenous balanced anesthesia. The response of adductor pollicis to train-of-four (TOF) stimulation of unlar nerve was monitored. The infusion rates of rocuronium and atracurium were adjusted to maintain T1/Tc ratio of 2%–10%. The total dose of each drug given during each of the three phases of OLT was recorded. Results: Rocuronium requirement, which were (0.468±0.167) mg/(kg·h) during the paleohepatic phase, decreased significantly during the anhepatic phase to (0.303±0.134) mg/(kg·h) and returned to the initial values at the neohepatic period ((0.429±0.130) mg/(kg·h)); whereas atracuruim requirements remained unchanged during orthotopic liver transplantation. Conclusions: This study showed that the exclusion of the liver from the circulation results in the significantly reduced requirement of rocuronium while the requirement of atracurium was not changed, which suggests that the liver is of major importance in the clearance of rocuronium. A continuous infusion of atracurium with constant rate can provide stable neuromuscular blockade during the three stages of OLT.
Similar content being viewed by others
References
Farman, J.V., Turner, J.M., Blanloeil, Y., 1986. Atracurium infusion in liver transplantation.Br. J. Anaesth.,58(Suppl 1) 96S-102S.
Fisher, D.M., Canfell, P.C., Fahey, M.R., Rosen, J.I., Rupp, S.M., Sheiner, L.B., Miller, R.D., 1986. Elimination of atracurium in humans: Contribution of Hofmann climination and ester hydrolysis versus organ-based elimination.Anesthesiology,65(1):6–12.
Fisher, D.M., Ramsay, M., Hein, T., Marcel, R.J., Sharma, M., Ramsay, K.J., Miller, R.D., 1997. Pharmacokinetics of rocuronium during the three stages of liver transplantation.Anesthesiology,86(6):1306–1316.
Gao, L., Ramzan, I., Baker, B., 2002. Rocuronium plasma concentrations during three phases of liver transplantation: Relationship with early postoperative graft liver function.Br. J. Anaesth.,88(6):764–770.
Gao, L., Ramzan, I., Baker, B., 2003. Rocuronium infusion requirements and plasma concentrations at constant levels of neuromuscular paralysis during three phases of liver transplantation.J. Clin. Anesth.,15(4):257–266.
Khuenl-Brady, K., Castagnoli, K.P., Canfell, P.C., Caldwell, J.E., Agostan, S., Miller, R.D., 1990. The neuromuscular blocking effects and pharmacokinetics of ORG9426 and ORG9616 in the cat.Anesthesiology,72(4):669–674.
Magorian, T., Wood, P., Caldwell, J., Fisher, D., Segredo, V., Szenohradszky, J., Sharma, M., Gruenke, L., Miller, R., 1995. The pharmacokinetics and neuromuscular effects of rocuronium bromide in patients with liver disease.Anesth. Analg.,80(4):754–759.
O'Kelly, B, Jayais, P., Veroli, P., Lhuissier, C., Ecoffy, C., 1991. Dose requirements of vecuronium, pancuronium, and atracurium during orthotopic liver transplantation.Anesth. Analg.,73(6):794–798.
Pitter, J.F., Tassonyi, E., Schopfer, C., Morel, D.R., Mentha, G., Fathi, M., Le Coultre, C., Steinig, D.A., Benakis, A., 1990. Plasma concentrations of laudanosine, but not of atracurium, are increased during the anhepatic phase of orthotopic liver transplantation in pigs.Anesthesiology,72(1):145–152.
Ward, S., Neill, E.A., 1983. Pharmacokinetics of atracurium in acute hepatic failure (with acute renal failure).Br. J. Anaesth.,55(12):1169–1172.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao-chuan, W., Liang, Z., Yin-yan, F. et al. Dose requirements of continuous infusion of rocuronium and atracurium throughout orthotopic liver transplantation in humans. J. Zheijang Univ.-Sci. B 6, 869–872 (2005). https://doi.org/10.1007/BF02840995
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02840995