Abstract
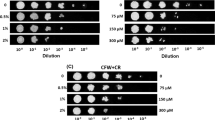
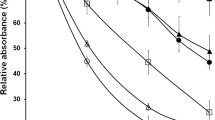
A strain ofFusarium oxysporum tolerated copper in the growth medium at concentrations up to 600 mg/L. The optimum growth was obtained at 200 mg Cu/L. The mycelium acquired a blue color in the presence of copper. The copper content of isolated cell walls obtained from mycelium grown in the presence of 600 mg Cu/L was 1.5 times higher than that of cell walls obtained from mycelium grown at 200 mg Cu/L and it contained 2.2 and 3.3% copper at 200 and 600 mg Cu/L, respectively. The amount of protein and total sugars increased in both the mycelium and its isolated cell walls in the presence of copper in the growth medium, chitin was also increased in the cell wall, reaching its maximum amount at 200 mg Cu/L— about 2.4 times higher than without copper. Most of amino acid concentrations in the cell wall were increased in the presence of 200 mg Cu/L and decreased above this concentration. Isoleucine, leucine, tyrosine, phenylalanine, and arginine showed the highest increase at this concentration. The altered cell walls obtained from mycelium grown at 200 and 400 mg Cu/L could rebind individual metals more than the control cell walls could. Rebinding of individual metals was in the order Zn>Fe>Ni>Cu>Co. Rebinding of copper by isolated cell walls depended on pH and temperature.
Similar content being viewed by others
References
Chen G.C., Johnson B.R.: Improved colorimetric determination of cell wall chitin in wood decay fungi.Appl. Environ.Microbiol.46, 13–16 (1983).
Dey S., Rao P.R.N., Bhattacharyya B.C., Bandyopadhyay M.: Sorption of heavy metals by four basidiomycetous fungi.Bioprocess Eng.12, 273–277 (1995).
Fourest E., Canal C., Roux J.C.: Improvement of heavy metal biosorption by mycelial dead biomasses (Rhizopus arrhizus, Mucor miehei andPenicillium chrysogenum) pH control and cationic activation.FEMS Microbiol.Rev.14, 325–332 (1994).
Galli U., Schuepp H., Brunold C.: Heavy metal binding by mycorrhizal fungi.Physiol. Plant92 364–368 (1994).
Garcia-Toledo A., Babich H., Stotzky G.: Training ofRhizopus stolonifer andCunninghamella blakesleeana to copper. Cotolerance to cadmium, cobalt, and lead.Can.J.Microbiol.31, 485–492 (1985).
Huang C., Huang C.P., Morehart A.L.: The removal of copper(II) from dilute aqueous solutions bySaccharomyces cerevisiae.,Water Res.24, 433–440 (1990).
Huang C., Huang C.P., Morehart A.L.: Proton competition in Cu(II) adsorption by fungal mycelia.Water Res.25, 1365–1375 (1991).
Hunsley D., Burnett J.H.: The ultrastructural architecture of the walls of some hyphal fungi.J. Gen. Microbiol.62, 203–218 (1970).
Jirku V.: Immobilized cell wall as a biospecific sorbent.Biotechnol. Lett.8, 639–642 (1986).
Jones D., Muehlchen A.: Effects of the potentially toxic metals, aluminium, zinc and copper on ectomycorrhizal fungi.J. Environ. Sci. Health A. Environ.Sci.Eng.29, 949–966 (1994).
Lyr H., Casperson G.: Pathological cell wall synthesis inMucor mucedo (L.)Fres. under the effect of fungicides and other components (in German). Abh. Akad. Wiss. DDR Abt. Math. Naturwiss. Tech. 1982 in Publ. 1983, 69–78;Chem. Abstr.99, 117704t (1982).
Motohiro F., Sunao Y., Shozo T.: Distribution of copper in the cells of heavy metal tolerant fungus,Penicillium ochro-chloron, cultured in concentrated copper medium.Agric.Biol.Chem.47, 1367–1369 (1983).
Muzzarelli R.A.A.:Chitin. Pergamon Press, Oxford 1974.
Muzzarelli R.A.A., Rochetti R.: The use of chitosan columns for removal of mercury from waters.J.Chromatogr.96, 115–121 (1974).
Muzzarelli R.A.A.: Removal of uranium from solutions and brines by a derivative of chitosan and ascorbic acid.Carbohydr. Polym.5, 85–89 (1985).
Oliferchuk V.P., Sukhomlin M.N., Zhdanova N.N.: Adsorption of heavy metal ions by some micromycetes from the sewage of the enterprise of precise machine building.Mikrobiol. Zh56, 65–70 (1994).
Patterson A., Kunst L., Bergman B., Roomans G.M.: Accumulation of aluminium byAnabaena cylindrica into polyphosphate granules and cell walls: an X-ray energy dispersive microanalysis study.J.Gen.Microbiol.131, 2545–2548 (1985).
Reedy L.H., Prasad M.N.V.: Heavy metal binding proteins/peptides: occurrence, structure, synthesis and functions. A review.Environ.Exp.Bot.30, 251–264 (1990).
Rodrigues R.K., Kelman D.J., Barton L.J.: Iron metabolism by an ectomycorrhizal fungus,Cenococcum graniforme.J.Plant Nutr.7, 459–468 (1984).
Schmit J.C., Edson C.M., Brody S.: Changes in glucosamine and galactosamine levels during conidial germination inNeurospora crassa.J. Bacteriol.122, 1062–1070 (1975).
Sietsma J.H., Wessels J.G.H.: Solubility of (1→3)-β-d/(1→6)-β-d-glucan in fungal walls: importance of presumed linkage between glucan and chitin.J.Gen.Microbiol.125, 209–212 (1981).
Subramanyam C., Venkateswerlu G., Rao S.L.N.: Cell wall composition ofNeurospora crassa under conditions of copper toxicity.Appl.Environ.Microbiol.46, 585–590 (1983).
Tsezos M.: The selective extraction of metals from solution by microorganisms.Can.Metall.24, 141–144 (1985).
Umbriet W.W., Burris R.H. Stauffer J.F., Cohen P.P., Johnse W.J., Leepage G.A., Patler V.R., Scheider W.C.:Manometric Techniques, a Manual Describing Methods Applicable to the Study of Tissue Metabolism, p. 239. Burgess Publ. Co. (1959).
Venkateswerlu G., Stotzky G.: Copper and cobalt alter the cell wall composition ofCunninghamella blakesleeana.Can.J.Microbiol.32, 654–662 (1986).
Venkateswerlu G., Stotzky G.: Binding of metals by cell walls ofCunninghamella blakesleeana grown in presence of copper or cobalt.Appl.Microbiol.Biotechnol.31, 619–625 (1989).
Volesky B.A., May-Phillips H.A.: Biosorption of heavy metals bySaccharomyces cerevisiae.Appl.Microbiol. Biotechnol.42, 797–806 (1995).
Wakatsuki T., Iba M., Imahara H.: Copper reduction by yeast cell wall materials and its role on copper uptake inDebaryomyces hansenii.J.Ferment.Technol.66, 257–265 (1988).
Wales D.S., Sagar B.F.: Recovery of metal ions by microfungal filters.J.Chem.Technol.Biotechnol.44, 345–356 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hefnawy, M.A., Razab, A.A. Alteration of cell-wall composition ofFusarium oxysporum by copper stress. Folia Microbiol 43, 453–458 (1998). https://doi.org/10.1007/BF02820790
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02820790