Abstract
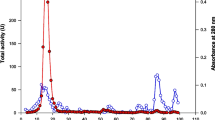
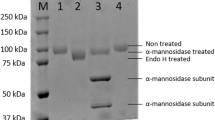
Raffinose-type galactose oligosaccharides constitute a substantial part (40%) of the soluble sugars present in soybean seeds and are responsible for flatulence following ingestion of soybean and other legumes. Enzymic hydrolysis of these oligosaccharides would improve the nutritional value of soybean milk.Aspergillus fumigatus produces substantial raffinose-hydrolysing and invertase activities when grown on wheat straw. Three proteins displaying maximal activity at pH 4.5–5.5 and 55–60°C and having molar mass of 66.8, 50.3 and 30.2 kDa were purified. Raffinose and sucrose were hydrolyzed with equivalent affinities by each protein. Nevertheless, theK m andV lim values determined for hydrolysis of sucrose by the 66.8 kDa enzyme differed from those determined with the 50.3 kDa protein. Glucose was produced when sucrose was the substrate. The three proteins hydrolyzed also stachyose but not melibiose, maltose, inulin or 4-nitrophenyl α-d-galactopyranoside.A. fumigatus enzymes may be candidates for processing of soybean milk to reduce its flatulence potential.
Similar content being viewed by others
References
Aslanidis C., Schmid K., Schmitt R.: Nucleotide sequence and operon structure of plasmid-borne genes mediating uptake and utilization of raffinose inEscherichia coli.J. Bacteriol.171, 6753–6763 (1989).
Aslanidis C., Schmid R.: Regulatory elements of the raffinose operon: nucleotide sequence of operator and repressor genes.J. Bacteriol.172, 2178–2180 (1990).
Bergmeyer H.U., Bernt E.: Determination of glucose with oxidase and peroxidase, pp. 1205–1215 in H.U. Bergmeyer (Ed.):Methods of Enzymatic Analysis, Vol. 3. New York, 1974.
Blum H., Beier H., Gross H.: Improved silver Verlag Chemie—Academic Press staining of plant proteins, RNA and DNA in polyacrylamide gels.Electrophoresis8, 93–99 (1987).
Bradford M.M.: A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle dye biding.Anal. Biochem.72, 680–685 (1976).
Burkardt H.J., Mattes R., Schimid K., Schmit R.: Properties of two conjugative plasmids mediating tetracycline resistance, raffinose catabolism and hydrogen sulfide production inEscherichia coli.Mol. Gen. Genet.166, 75–84 (1978).
Cavazzoni V., Adami A., Craveri R.: α-Galactosidase from the yeastCandida javanica.Appl. Microbiol. Biotechnol.26, 555–559 (1987).
Laemmli U.K.: Cleavage of structural proteins during the assembly of head of bacteriophage T4.Nature227, 680–683 (1970).
Miller G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar.Anal. Chem.31, 426–428 (1956).
Moriguchi T., Sanada T., Yamaki S.: Properties of invertase purified from peach fruits.Phytochemistry30, 95–97 (1990).
Moser M., Menz G., Blaser K., Crameri R.: Recombinant expression and antigenic properties of a 32-kilodalton extracellular alkaline protease, representing possible virulence factor formAspergillus fumigatus.Infect. Immun.62, 936–942 (1994).
Mukherjee K., Sengupta S.: Purification and properties of nonspecific β-fructofuranosidase (inulinase) from the mushroomPanœolus papillonaceus.Can. J. Microbiol.33, 520–524 (1987).
Price K.R., Lewis J., Wyatt G.M., Fenwick G.R.: Flatulence-causes, relation to diet and remedies.Nahrung32, 609–626 (1988).
Quiroga E.N., Vattuone M.A., Sampietro A.R.: Purification and characterization of the invertase fromPycooporus sanguineus.Biochim. Biophys. Acta125, 75–80 (1995).
de Rezende S.T., Felix C.R.: Raffinose-hydrolyzing ofAspergillus fumigatus.Biotechnol. Letters19, 217–220 (1997).
Rodriguez J., Perez J.A., Ruiz T., Rodriguez L.: Characterization of the invertase fromPichia anomala.Biochem. J.306, 235–239 (1995).
Rojo H.P., Vattuone M.A., Sampietro A.R.: Invertase fromSchizophyllum commune.Phytochemistry37, 119–123 (1994).
Santos R.M.B., Firmino A.A.P., Felix C.R.: Keratinolytic activity ofAspergillus fumigatus.Cur. Microbiol.33, 364–370 (1996).
Schmid K., Ritschewald S., Schmitt R.: Relationships among raffinose plasmids determined by the immunochemical cross-reaction of their α-galactosidase.J. Gen. Microbiol.114, 477–481 (1979).
Yanase H., Iwata M., Kita K., Kato N., Tonomura K.: Purification, crystallization, and characterization of the extracellular invertase fromZymomonas mobilis.J. Ferment. Bioeng.79, 367–369 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Rezende, S.T., Felix, C.R. Production and characterization of raffinose-hydrolysing and invertase activities ofAspergillus fumigatus . Folia Microbiol 44, 191–195 (1999). https://doi.org/10.1007/BF02816241
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02816241