Abstract
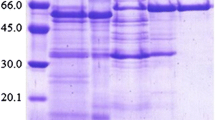
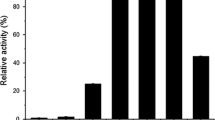
The high-molar mass from of β-glucosidase fromAspergillus niger strain NIAB280 was purified to homogeneity with a 46-fold increase in purification by a combination of ammonium sulfate precipitation, hydrophobic interaction, ion-exchange and gel-filtration chromatography. The native and subunit molar mass was 330 and 110 kDa, respectively. The pH and temperature optima were 4.6–5.3 and 70°C, respectively. TheK m andk cat for 4-nitrophenyl β-d-glucopyranoside at 40°C and pH 5 were 1.11 mmol/L and 4000/min, respectively. The enzyme was activated by low and inhibited by high concentrations of NaCl. Ammonium sulfate inhibited the enzyme. Thermolysin periodically inhibited and activated the enzyme during the course of reaction and after 150 min of proteinase treatment only 10% activity was lost with concomitant degradation of the enzyme into ten low-molar-mass active bands. When subjected to 0–9 mol/L transverse urea-gradient-PAGE for 105 min at 12°C, the nonpurified β-glucosidase showed two major bands which denatured at 4 and 8 mol/L urea, respectively, with half-lives of 73 min.
Similar content being viewed by others
References
Bennett J.W., Klich M.A.: Industrial enzymes fromAspergillus species, pp. 175–180 inAspergillus: Biology and Industrial Applications (J.W. Bennett, M.A. Klich, Eds.). Butterworth-Heinemann, Boston 1992.
Coughlan M.P.: Staining techniques for the detection of the individual components of cellulolytic enzyme systems.Methods Enzymol. 160, 135–144 (1988).
Deshpande V., Eriksson K.E.: 1,4-β-Glucosidases ofSporotrichum pulverulentum.Methods Enzymol. 160, 415–424 (1988).
Esen A.: β-Glucosidases, over review, pp. 1–14 inβ-Glucosidases, Biochemistry and Molecular Biology (A. Esen, Ed.), American Chemical Society, Washington (DC) 1993.
Esen A., Gungor G.: Stability and activity of plant and fungal β-glucosidases under denaturing conditions, pp. 214–239 inβ-Glucosidases: Biochemistry and Molecular Biology (A. Esen, Ed.), American Chemical Society, Washington (DC) 1993.
Goldenberg D.P.: Analysis of protein conformation by gel electrophoresis, pp. 225–250 inProtein Structure: a Practicle Approach (T.E. Creighton, Ed.), IRL Press, Oxford (UK) 1989.
Himmel M.E., Adney W.S., Fox J.W., Mitchell D.J., Baker J.O.: Isolation and characterization of two forms of β-d-glucosidase fromAspergillus niger.Appl. Biochem. Biotechnol. 39/40, 213–225 (1993).
Hoh Y.K., Yeoh H.H., Tan T.K.: Isolation and characterization of β-glucosidase fromAspergillus nidulans mutant USDB 1183.World J. Microbiol. Biotechnol. 9, 555–558 (1993).
Kvesitadze G.I., Svanidze R.S., Tsuprun V.L., Nizharadze D.N., Chirgadze L.T., Buachidze T.S.: Quaternary structure and properties of β-glucosidase isolated from a thermophilic culture ofAspergillus wentii.Bioorg. Khim. 16, 881–888 (1990).
Laemmli U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature 227, 680–685 (1970).
McCleary B.V., Harrington J.: Purification of β-glucosidase fromAspergillus niger.Methods Enzymol. 160, 575–583 (1988).
Rashid M.H., Siddiqui K.S.: The stability of extracellular β-glucosidase fromAspergillus niger is significantly enhanced by noncovalently attached polysaccharides.Folia Microbiol. 41, 341–346 (1996).
Sadana J.C., Patil R.V., Shewale J.G.: β-d-Glucosidases fromSclerotium rolfsii.Methods Enzymol. 160, 424–431 (1988).
Sanyal A., Kundu R.K., Dube S., Dube D.K.: Extracellular cellulytic enzyme system ofAspergillus japonicus: purification and characterization of an inducible extracellular β-glucosidase.Enzyme Microb. Technol. 10, 91–99 (1988).
Siddiqui K.S., Rashid M.H., Rajoka M.I.: Kinetic analysis of the active site of an intracellular β-glucosidase fromCellulomonas biazotea.Folia Microbiol. 42, 53–58 (1997).
Sigma Technical Bulletin, no. MRK-137 (1986).
Unno T., Ide K., Yazaki T., Tanaka Y., Nakakuki T., Okada G.: High recovery purification and some properties of a β-glucosidase fromAspergillus niger.Biosci. Biotech. Biochem. 57, 2172–2173 (1993).
Vodjdani G., Dizet P.L., Petek F.: Purification et proprietes de deux (1→4)-β-d-glucosidases d'Aspergillus roseus.Carbohydr. Res. 236, 267–279 (1992).
Wood T.M., Bhat K.M.: Methods for measuring cellulase activities.Methods Enzymmol. 160, 87–112 (1988).
Ximenes E.A., Felix C.R., Ulhoa C.J.: Production of cellulases byAspergillus fumigatus and characterization of one β-glucosidase.Curr. Microbiol. 32, 119–123 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rashid, M.H., Siddiqui, K.S. Purification and characterization of a β-glucosidase fromAspergillus niger . Folia Microbiol 42, 544–550 (1997). https://doi.org/10.1007/BF02815462
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02815462