Abstract
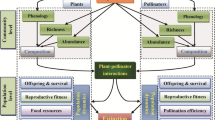
The exchange of ideas and information between vegetation ecology and pollination ecology is relatively restricted, yet both fields have devised methods to detect the structure of species assemblages and communities. To promote the exchange of ideas between fields I compare approaches, concepts, and problems faced by researchers working in each area. Both vegetative and reproductive interactions may generate assemblage structure through ecological sorting or through character displacement. Vegetative interactions may lead to assemblage organization more often by ecological sorting and reproductive interactions more often by character displacement. Vegetative interactions generally operate over shorter temporal and smaller spatial scales than reproductive interactions and may be affected more strongly by temporal and spatial heterogeneity in abiotic and biotic environments. These differences affect how the concept of ecological niche should be applied to plants. The Hutchinsonian concept of niche needs to be significantly modified before it can be usefully applied to plants.
Null models are a valuable tool for investigating both vegetative and reproductive structuring of plant assemblages; however, the procedures followed in the application of null models need further refinement. The appropriate formulation of the null model may require information that is unavailable, hence multiple models may have to be employed to “bracket” conclusions. The literature on pollination community ecology demonstrates that difficult decisions must be made about the likely processes that have generated the structure being tested, the relevant definition of sympatry, how guid membership should be defined and employed, and what constraints should be incorporated into the null model to impose realism. Differences in these decisions will affect the outcome of the analysis. While top-down studies of pattern have numerous advantages, they usually cannot identify the process(es) that have generated the patterns. Bottom-up, experimental studies can be useful for identifying the processes, but they can rarely be used to assess the structure of an entire natural assemblage. The optimal approach to studying assemblage structure is to detect patterns with top-down analysis and use experiments to identify the processes that generate and maintain the patterns.
Similar content being viewed by others
References
Armbruster W.S. (1980): Pollination relationships between four sympatric species ofCollinsia (Scrophulariaceae).—Bot. Soc. Amer. Misc. Ser. Publ. 158:8.
Armbruster W.S. (1985): Patterns of character divergence and the evolution of reproductive ecotypes ofDalechampia scandens (Euphorbiaceae).—Evolution 39: 733–752.
Armbruster W.S. (1986): Reproductive interactions between sympatricDalechampia species: are natural assemblages “random” or organized?.—Ecology 67: 522–533.
Armbruster W.S., Edwards M.E. &Debevec E.M. (1994): Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium).—Ecology 75: 315–329.
Armbruster W.S. &McGuire A.D. (1991): Experimental assessment of reproductive interactions between sympatricAster andErigeron (Asteraceae) in interior Alaska.—Amer. J. Bot. 78: 1449–1457.
Ashton P.S., Givnish T.J. &Appanah S. (1988): Staggered flowering in theDipterocarpaceae: new insights into floral induction and the evolution of mast fruiting in the aseasonal tropics.—Amer. Naturalist 132: 44–66.
Bengtsson J., Fagerström T. &Rydin H. (1994): Competition and coexistence in plant communities. —Trends Ecol. Evol. 9: 246–250.
Campbell D.C. (1985): Pollinator sharing and seed set ofStellaria pubera: competition for pollination.— Ecology 66: 554–563.
Campbell D.C. &Motten A. (1985): The mechanism of competition for pollination between two forest herbs.—Ecology 66: 554–563.
Chesson P. (1991): A need for niches?.—Trends Ecol. Evol. 6: 26–28.
Cole B.J. (1981): Overlap, regularity, and flowering phenologies.—Amer. Naturalist 117: 993–997.
Diamond J. &Gilpin M. (1982): Examinations of the null model of Connor and Simberloff for species co-occurrences on islands.—Oecologia 52: 64–74.
Fagerström T. (1988): Lotteries in communities of sessile organisms.—Trends Ecol. Evol. 3: 303–306.
Feinsinger P. (1987): Effects of plant species on each other’s polliation: is community structure influenced? —Trends Ecol. Evol. 2: 123–126.
Flemming T.H. (1985): Coexistence of five sympatricPiper (Piperaceae) species in a tropical dry forest.— Ecology 66: 688–700.
Flemming T.H. &Partridge B.L. (1984): On the analysis of phenological overlap.—Oecologia 62: 344–350.
Gleeson S.K. (1981): Character displacement in flowering phenologies.—Oecologia 51: 294–295.
Gotelli N.J. (1991): Metapopulation models: the rescue effect, the propagule rain, and the core-satellite hypothesis.—Amer. Naturalist 138: 768–776.
Grant P.R. &Abbott I. (1980): Interspecific competition, island biogeography and null hypothesis.— Evolution 34: 332–341.
Grubb P.J. (1977): The maintenance of species richness in plant communities. The importance of the regeneration niche.—Biol. Rev. 52: 107–145.
Hanski I. (1982): Dynamics of regional distribution: the core and satellite species hypothesis.—Oikos 38: 210–221.
Harper J.L. (1967): A Darwinian approach to plant ecology.—J. Ecol. 55: 247–270.
Harvey P.H., Colwell R.K., Silvertown J.W. &May R.M. (1983): Null models in ecology. —Annual Rev. Ecol. Syst. 14: 189–211.
Hocking B. (1968): Insect-flower associations in the high Arctic, with special reference to nectar.—Oikos 19: 359–388.
Hubbell S.P. &Foster R.B. (1986): Biology, chance, and history and the structure of tropical rain forest tree communities.—In:Diamond J. &Case T.J. [eds.]: Community ecology, Harper and Row, New York, pp. 314–329.
Hurlbert S.H. (1970): Flower number, flowering time, and reproductive isolation among ten species ofSolidago (Compositae).—Bull. Torrey Bot. Club 97: 189–193.
Hutchinson G.E. (1957): Concluding remarks.—Cold Spring Harbor Symp. Quant. Biol. 22: 415–427.
Janzen D.H. (1971): Euglossine bees as long-distance pollinators for tropical plants.—Science 171: 203–205.
Janzen D.H. (1985): Male dogs have fitness.—Biotropica 17: 205.
Kjellsson G. (1985): Seed fall and phenological overlap in a guild of ant-dispersed herbs.—Oecologia 68: 140–146.
Klimeš L. (1995): Small-scale distribution of species richness in a grassland (Bílé Karpaty Mts., Czech Republic).—Folia Geobot. Phytotax. 30: 499–510.
Klimeš L., Jongepier J.W., &Jongepierová I. (1995): Variability in species richness and guild structure in two species-rich grasslands.—Folia Geobot. Phytotax. 30: 243–253.
Kochmer J.P. &Handel S.N. (1986): Constraints and competition in the evolution of flowering phenology. —Ecol. Monogr. 56: 303–325.
Lack D. (1947): Darwin’s finches.—Cambridge University Press, Cambridge.
Lepš J. (1995): Variance deficit is not reliable evidence for niche limitation.—Folia Geobot. Phytotax. 30: 455–459.
Levin D.A. (1970): Reinforcement of reproductive isolation: plants versus animals.—Amer. Naturalist 104: 571–581.
Lewis H. (1961): Experimental sympatric populations ofClarkia.—Amer. Naturalist 104: 455–467.
van der Maarel E. &Sykes M.T. (1993): Small-scale plant species turnover in a limestone grassland: the carousel model and some comments on the niche concept.—J. Veg. Sci. 4: 179–188.
McGuire A.D. (1989): The organization of flowering times of the insect-pollinated flora of south-facing bluffs in interior Alaska: evaluation of the reproductive-interference hypothesis—Ph.D. dissertation, Univ. Alaska Fairbanks, USA.
McGuire A.D. (1993): Interactions for pollination between two synchronously bloomingHedysarum species (Fabaceae) in Alaska.—Amer. J. Bot. 80: 147–152.
McGuire A.D. &Armbruster W.S. (1991): An experimental test for reproductive interactions between two sequentially bloomingSaxifraga species.—Amer. J. Bot. 78: 214–219.
Mosquin T. (1971): Competition for pollinators as a stimulus for the evolution of flowering time.—Oikos 22: 398–402.
Murdy W.H., Johnson T.M. &Wright V.K. (1970): Competitive replacement ofTalinum mangesii byT. teretifolium in granite outcrop communities of Georgia.—Bot. Gaz. 131: 186–192.
Murray G.K., Feinsinger P., Busby W.H., Linhart Y., Beach J.H. &Kinsman S. (1987): Evaluation of character displacement among plants in two tropical pollination guilds.—Ecology 68: 1283–1293.
Palmer M.W. (1987): Variability in species richness within Minnesota oldfields: a use of the variance test.— Vegetatio 70: 61–64.
Palmer M.W. &van der Maarel E. (1995): Variance in species richness, species association, and niche limitation.—Oikos 73: 203–213.
Parrish J.A.D. &Bazzaz F. (1979): Difference in pollination niche relationships in early-and late-successional plant communities.—Ecology 60: 597–610.
Pianka E.R. (1974): Niche overlap and diffuse competition.—Proc. Natl. Acad. USA 71: 2141–2145.
Pleasants J.M. (1980): Competition for bumblebee pollinators in Rocky Mountain plant communities.— Ecology 61: 1446–1459.
Pleasants J.M. (1990): Null-model tests for competitive displacement: the fallacy of not focusing on the whole community.—Ecology 71: 1078–1084.
Pojar J. (1974): Reproductive dynamics of four plant communities of southwestern British Columbia.—Canad. J. Bot. 52: 1819–1834.
Poole R.W. &Rathcke B.J. (1979): Regularity, randomness, and aggregation in flowering phenologies.— Science 203: 470–471.
Rabininowitz D., Rapp J.K., Sork V.L., Rathcke B.J., Reese G.A. &Weaver J.C. (1981): Phenological properties of wind and insect-pollinated prairie plants.—Ecology 62: 49–56.
Rathcke B.J. (1988): Flowering phenology in a shrub community competition and constraint.—J. Ecol. 76: 975–994.
Reader R.J. (1975): Competitive relationships of some bog ericads for major insect pollinators.—Canad. J. Bot. 53: 1300–1305.
Robertson C. (1895): The philosophy of flower seasons, and the phenological relations of the entomophilous flora and anthophilous insect fauna.—Amer. Naturalist 29: 97–117.
Schoener T.W. (1970): Non-synchronous spatial overlap of lizards in patchy habitatas.—Ecology 51: 408–418.
Shimida A. &Ellner S. (1984): Coexistence of plant species with similar niches.—Vegetatio 58: 29–55.
Silvertown J. &Law R. (1987): Do plants need niches? Some recent developments in plant community ecology.—Trends Ecol. Evol. 2: 24–26.
Snow D.W. (1965): A possible selective factor in the evolution of fruiting seasons in tropical forest.—Oikos 15: 274–281.
Stiles F.G. (1977): Co-adapted competitors: the flowering seasons of hummingbird-pollinated plants in a tropical forest.—Science 198: 1170–1178.
Stiles F.G. (1979): Reply to Poole and Rathcke.—Science 203: 1177–1178.
Tilman D. (1982): Resource competition and community structure.—Princeton Univ. Press, Princeton, NY.
Tilman D. (1988): Dynamics and structure of plant communities.—Princeton Univ. Press, Princeton, NY.
Toft C.A. &Shea P.J. (1983): Detecting community-wide patterns: estimating power strengthens statistical inference.—Amer. Naturalist 122: 618–625.
Waser N.M. (1978): Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers.—Ecology 59: 934–944.
Waser N. M. (1983): Competition for pollination and floral character differences among sympatric plant species: a review of the evidence.—In:Jones C.E. &Little R.J. [eds.]: Handbook of experimental pollination ecology.—Van Nostrand Reinhold, New York, pp. 277–293.
Watkins A.J. &Wilson J.B. (1992): Fine-scale community structure of lawns.—J. Ecol. 80: 15–24.
Whalen M.D. (1978): Reproductive character displacement and floral diversity inSolanum sect.Androceras. —Syst. Bot. 3: 77–86.
Wheelwright N.T. (1985): Competition for dispersers, and the time of flowering and fruiting in a guild of tropical trees.—Oikos 44: 465–477.
Wilson J.B. (1995): Testing for community structure: a Bayesian approach.—Folia Geobot. Phytotax. 30: 461–469.
Wilson J.B. & Gitay H. (1995a): Community structure and assembly rules in a dune slack: Variance in richness, guild proportionality, biomass constancy and dominance/diversity relations.—Vegetatio (in press).
Wilson J.B. &Gitay H. (1995b): Limitations to species coexistence: evidence for competition from field observations, using a patch model.—J. Veg. Sci. 6: 369–376.
Wilson J.B. & Whittaker R.J. (1995): Assembly rules demonstrated in a saltmarsh community.—J. Ecol. 83 (in press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Armbruster, W.S. The origins and detection of plant community structure: Reproductive versus vegetative processes. Folia Geobot 30, 483–497 (1995). https://doi.org/10.1007/BF02803978
Issue Date:
DOI: https://doi.org/10.1007/BF02803978