Abstract
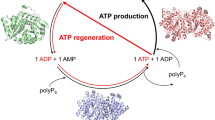
Syntheses of adenosine 5′-triphosphate (ATP) from adenosine 5′-monophosphate (AMP) and ribavirin 5′-triphosphate (RTP) from ribavirin 5′-monophosphate (RMP) (1) were performed using enzymes as catalysts. Synthesis of ATP is based on acetyl phosphate as the phosphate donor, and acetate kinase (Bacillus stearothermophilus, EC 2.7.2.1), adenylate kinase (porcine muscle, EC 2.7.4.3), and inorganic pyrophosphatase (yeast, EC 2.6.1.1) as the catalysts. Three reactions on a 150-mmol scale provided ATP as its barium salt in 82% yield and 67% purity. Synthesis of RTP used phosphoenol pyruvate (PEP) as the phosphate donor, and pyruvate kinase (rabbit muscle, EC 2.7.1.40) and adenylate kinase (rabbit muscle) as the catalysts. A gram-scale reaction provided RTP as its barium salt in 93% yield and 97% purity. This work demonstrates the utility of the autoxidationresistant acetate kinase fromB. stearothermophilus, the value of pyrophosphatase in controlling the level of pyrophosphate in the reactions and the ability of adenylate kinase to accept at least one substrate other than a derivative of adenosine.
Similar content being viewed by others
References
This research was supported by the National Institutes of Health, Grant GM 30367.
Whitesides, G. M. and Wong, C.-H. (1985),Angew. Chem. Int. Ed. Eng. 24, 617.
Jones, J. B. (1986),Tetrahedron 42, 3351.
Crans, D. C, Kazlauskas, R. J., Hirschbein, B. L., Wong, C. H., Abril, O., and Whitesides, G. M.Methods Enzymol, in press.
Crans, D. C. and Whitesides, G. M. (1985),J. Am. Chem. Soc. 107, 7008;ibid., 7019.
Wong, C.-H., Haynie, S. L., and Whitesides, G. M. (1983),J. Am. Chem. Soc. 105, 115.
Hirschbein, B. L., Mazenod, F. P., and Whitesides, G. M. (1982),J. Org. Chem. 47, 3765.
Wong, C.-H., McCurry, S. D., and Whitesides, G. M. (1980),J. Am. Chem. Soc. 102, 7938.
Ladner, W. F. and Whitesides, G. M. (1985),J. Org. Chem. 50, 1076.
Dixit, V. M. and Poulter, C. D. (1984),Tetrahedron Lett. 25, 4055.
Scheit, K. H. (1980),Nucleotide Analogs; Wiley, New York, chapter 6.
Tani, Y., Yonehara, T., Mitani, Y., and Yamada, H. (1984),J. Biotechnol. 1, 119; Tani, Y., Mitani, Y., and Yamada, H. (1984),J. Ferment. Technol. 62, 99.
Tani, Y., Mitani, Y., and Yamada, H. (1984),Agric. Biol. Chem. 48, 431.
Asada, M., Morimoto, K., Nakanishi, K., Matsuno, R., Tanaka, A., Kimura, A., and Kamikubo, T. (1979),Agric. Biol. Chem. 43, 1773.
Asada, M., Nakanishi, K., Matsuno, R., Kariya, Y., Kimura, A., Kamikubo, T. (1978),Agri. Biol. Chem. 42, 1533.
Nakajima, H., Nagata, K., Kondo, H., Imahori, K. (1984),J. Appl. Biochem. 6, 19; Kondo, H., Tomioka, I., Nakajima, H., and Imahori, K.ibid., 29.
Leuchs, H.-J., Lewis, J. M., Rios-Mercadillo, V. M., and Whitesides, G. M. (1979),J. Am. Chem. Soc. 101, 5829.
Baughn, R. L., Actalsteinsson, O., and Whitesides, G. M. (1978),J. Am. Chem. Soc. 100, 304.
Rose, I. A. (1962), inThe Enzymes, 2nd ed., Boyer, P. D., Lardy, H., and Myrback, K., eds., Academic, New York, vol. 6, chapter 7.
Lewis, J. M., Haynie, S. L., and Whitesides, G. M. (1979),J. Org. Chem. 44, 864; Whitesides, G. M., Siegel, M., and Garett, P. (1975),J. Org. Chem. 40, 2516.
Kazlauska, R. J. and Whitesides, G. M. (1985),J. Org. Chem. 50, 1069.
Crans, D. C. and Whitesides, G. M. (1983),J. Org. Chem. 26, 3130.
Nakajima, H., Suzuki, K., and Imahari, K. (1978),J. Biochem. 84, 193;Methods Enzymol. 90, 179.
Noda, L. InThe Enzymes, 3rd ed.; Boyer, P. D., ed., Academic, New York, 1973; vol. 8, part A, chapter 8.
Sidwell, R. W., Huffman, J. H., Robins, R. K., Khare, G. P., Allen, L. B., Witkowski, J. T., and Ronins, R. K. (1972),Science 177, 705.
Witkowski, J. T., Robins, R. K., Sidwell, R. W., and Simon, L. N. (1972),J. Med. Chem. 15, 1150.
Witkowski, J. T., Robins, R. K., Khare, G. P., and Sidwell, R. W. (1973),J. Med. Chem. 16, 935.
Allen, L. B., Boswell, K. H., Khwaja, T. A., Meyer, R. B., Jr., Sidwell, R. W., and Witkowski, J. T. (1978),J. Med. Chem. 21, 742.
Pollak, A., Blumenfeld, H., Wax, M., Baughn, R. L., and Whitesides, G. M. (1980),J. Am. Chem. Soc. 102, 6324; Pollak, A., Baughn, R. L., Adalsteinsson, O., and Whitesides, G. M. (1978),J. Am. Chem. Soc. 100, 302.
Methods of Enzymatic Analysis, 3rd ed., Bergmeyer, H. S., Bergmeyer, J., Grassl, M., eds., Verlag Chemie. Weinheim, 1983; vol. 2, pp. 333–345;ibid.; vol. 7, pp. 346–357 and 365–370.
Bailey, K. and Webb, E. C. (1944),Biochem. J. 38, 394.
The low purity of ATP reflects the use of an excess of AcP (3 equiv per equiv of nucleotides) and 90% conversion of AMP to ATP. The major impurities were inorganic phosphate, ADP, and AMP.
Bauer, P. I. and Varady, G. (1978),Anal. Biochem. 91, 613.
Adenylate kinase also accepts CMP as a substrate, howbeit with low vmax. This activity provides the basis for a synthesis of CTP: Simon, E. S., Bednarski, M. D., and Whitesides, G. M. (1988),Tetrahedron Lett. 29, 1123; Simon, E. S., Bednarski, M. D. and Whitesides, G. M.,J. Am. Chem. Soc., in press.
Bednarski, M. D., Chenault, H. K., Simon, E. S., and Whitesides, G. M. (1987),J. Am. Chem. Soc. 109, 1283.
Josse, J. (1966),J. Biol. Chem. 241, 1938.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, MJ., Whitesides, G.M. Enzyme-catalyzed synthesis of nucleoside triphosphates from nucleoside monophosphates. Appl Biochem Biotechnol 16, 95–108 (1987). https://doi.org/10.1007/BF02798359
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02798359
Index entries
- Acetate kinase, catalyst for synthesis of nucleoside triphosphates
- adenylate kinase, catalyst for synthesis of nucleoside triphosphates
- ATP, synthesis from AMP
- enzymes, catalysts in organic synthesis
- nucleoside triphosphates, synthesis from monophosphates
- ribavirin 5′-triphosphate, synthesis from ribavirin 5′-monophosphate