Summary
Conclusion
A new, stable, transplantable human pancreatic cancer xenograft (PZX-5) model has been established in CBA immunosuppressed mice.
Background
Numerous human pancreatic carcinomas have been successfully transplanted into athymic nude mice. However, artificially immunosuppressed animals have rarely been used as recipients. Because this model system proved to be reliable for hosting many human malignancies at our institute, successive xenotransplantations of a ductal adenocarcinoma have been carried out.
Method
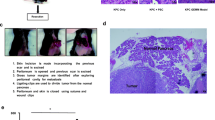
Immunosuppression of CBA/CA mice was achieved by thymectomy, whole-body irradiation and bone-marrow reconstruction. Tumor fragments were subcutaneously implanted from a well/moderately differentiated ductal pancreatic adenocarcinoma and serially transplanted for more than 20 mo. The xenografted tumors were characterized using morphological, immunohistochemical, biochemical, and flow cytometric methods.
Results
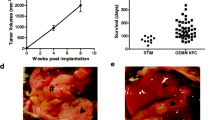
During the serial transplantations, the neoplasm maintained its original morphological-pathobiological characteristics. It produced a large amount of mucin and expressed carcinoembryonic antigen (CEA). Neither the mitotic activity nor the degree of differentiation was altered, and CEA was permanently detected. Flow cytometric DNA analysis revealed an aneuploid pattern (DNA index 1.45±0.03), which has remained within the same range during xenograftings. The doubling time in an in vitro system proved to be 18 h. The human character has been well preserved even 9 mo posttransplantation, as was evidenced by LDH-isoenzyme electrophoresis. The results indicate that the thymectomized—whole-body irradiated—bone-marrow reconstructed immunosuppressed mice are also appropriate hosts for pancreatic cancer xenografts.
Similar content being viewed by others
References
Janes RAH, Niederhuber JE, Chmiel JS, Winchester DP, Ocwieja KC, Karnell LH, Clive RE, Menck R. National patterns of care for pancreatic cancer. Results of a survey by the Commission on Cancer.Ann Surg 1996; 223: 261–272.
Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinocopathologic analysis of 5-year survivors.Ann Surg 1996; 223: 273–279.
Sperti C, Bonadimani B, Pasquali C, Piccoli A, Cappellazzo F, Rugge M, Pedrazzoli S. Ductal adenocarcinoma of the pancreas: clinicopathologic features and survival.Tumori 1993; 79: 325–330.
Scarpelli DG, Rao MS, Reddy JK. Studies of pancreatic carcinogenesis in different animal models.Environ Hlth Persp 1984; 56: 219–227.
Rao MS, Reddy JK. Induction and differentiation of exocrine pancreatic tumors in the rat.Exp Pathol 1985; 28: 67–87.
Pour P, Althoff J, Krüger FW, Mohr U. A potent pancreatic carcinogen in Syrian hamsters: N-nitrosobis(2-oxopropyl)amine.J Natl Cancer Inst 1977; 58: 1449–1453.
Scarpelli DG, Rao S. Transplantable ductal adenocarcinoma of the Syrian hamster pancreas.Cancer Res 1979; 39: 452–458.
Rao MS. Animal models of exocrine pancreatic carcinogenesis.Cancer Metast Rev 1987; 6: 665–676.
Mattern J, Bak M, Hahn E, Volm M. Human tumor xenografts as model for drug testing.Cancer Metast Rev 1988; 7: 263–284.
Kopf-Maier P, Kestenbach U. Host-supplied connective tissue as a guide for proliferating tumor cells in human tumor xenografts.Acta Anat Basel 1989; 135: 289–295.
Pickard RG, Cobb LM, Steel GG. The growth kinetics of xenografts of human colorectal tumors in immune-deprived mice.Br J Cancer 1975; 31: 36–45.
English HF, Kloszewski ED, Valentine EG, Santen RJ. Proliferative response of Dunning R3327H experimental model of prostatic adenocarcinoma to conditions of androgen depletion and repletion.Cancer Res 1986; 46: 839–844.
Szendrôi M, Vajta G, Kovács L, Schaff Z, Lapis K. Polarization colours of collagen fibers: a sign of collagen production activity in fibrotic process.Acta Morphol Hung 1984; 32: 47–55.
Keepers YP, Pizao FE, Peters GJ, Vanarkotte J, Winograd B, Pinedo HM. Comparison of the sulforhodamine-B protein and tetrazolium (MMT) assays for in vitro chemosensitivity testing.Eur J Cancer 1991; 27: 879–900.
Martin A, Clyness M. Comparison of 5 microplate colorimetric assays for in vitro cytotoxicity testing and cell proliferation assays.Cytotechnology 1993; 11: 49–58.
Lapis K, Bocsi J, Lapis P, Thorgeirsson UP. Flow cytometric DNA-ploidity and proliferative activity of diethylnitrosamine—induced hepatocellular carcinoma and pulmonary metastases in monkeys.Hepatology 1995; 22: 952–961.
Montes DeOca F, Macy ML, Shannon JE. Isoenzyme characterization of animal cell cultures.Proc Soc Exp Biol Med 1969; 132: 462–469.
Yagamata S, Ito Y, Tanaga R, Shimizu G. Gelatinases of metastatic cell lines of murine colonic carcinoma as detected by substrate-gel electrophoresis.Biochem Biophys Res Comm 1988; 151: 158–162.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature 1970; 227: 680–685.
Zalatnai A, Schally AV. Responsiveness of the hamster pancreatic cancer to treatment with microcapsules of D-Trp-6-LH-RH and somatostatin analog RC-160.Int J Pancreatol 1989; 4: 149–160.
Szende B, Srkalovic G, Groot K, Lapis K, Schally AV. Regression of nitrosamine-induced pancreatic cancers in hamsters treated with luteinizing hormone-releasing hormone antagonists or agonists.Cancer Res 1990; 50: 3716–3721.
Visser CJT, Van Garderen-Hoetmer A, Klun JGM, Foekens JA, Woutersen RA. Effects of Sandostatin, alone and in combination with surgical castration, on pancreatic carcinogenesis in rats and hamsters.Int J Cancer 1996; 65: 827–832.
Steel GG, Courtenay VD, Peckham MJ. The response to chemotherapy of a variety of human tumour xenografts.Br J Cancer 1983; 47: 1–13.
Chu MY, Zuckerman LB, Sato S, Crabtree GW, Bogden AE, Lim MI, Klein RS. 9-Deazaadenosine a new potent antitumor agent.Biochem-Pharmacol 1984; 33: 1229–1234.
Kobari M, Hisano H, Marsuno S, Sato T, Kan M, Tachibana T. Establishment of six human pancreatic cancer cell lines and their sensitivities to anti-tumor drugs.Tohoku J Exp Med 1986; 150: 231–248.
Rapaport E. Experimental cancer therapy in mice by adenine nucleotides.Eur J Cancer Clin Oncol 1988; 24: 1491–1497.
Klapdor R, Franke N, Bahlo M. Combined therapy of xenografts of human pancreatic carcinomas with rTNF-alpha and mitomycin C.Onkologie 1989; 12: 143–147.
Stanbridge EJ, Perkins FT. Tumourgenicity testing in immunosuppressed mice: advantages and disadvantages.Dev Biol Stand 1976; 37: 211–217.
Schmidt M, Deschner EE, Thaler HT, Clements L, Good RA. Gastrointestinal cancer studies in the human to nude mouse heterotransplant system.Gastroenterology 1977; 72: 829–837.
Davies G, Duke D, Grant AG, Kelly SA, Hermon-Taylor J. Growth of human digestive-tumour xenografts in athymic nude rats.Br J Cancer 1981; 43: 53–58.
Kubota T, Yamaguchi H, Wantanabe M, Yamamoto T, Takahara T, Takeuchi T, Furukawa T, Kase S, Kodaira S, Ishibiki K. Growth of human tumor xenografts in nude mice and mice with severe combined immunodeficiency (SCID).Surg Today 1993; 23: 375–377.
Hay JH, Morten JE, Clarke B, Swinton J. The suitability of immunosuppressed mice kept in a standard animal unit as recipients of human tumour xenografts.Lab Anim 1985; 19: 119–122.
Kopper L, Van Hanh T, Hegedus C, Lapis K. Growth of human colorectal tumor xenografts in immunosuppresed mice reconstituted with normal cells.Exp Cell Biol 1981; 49: 141–147.
Gyorfi T, Timár J, Lapis K. Liver-metastic human colon carcinoma in immunosuppressed mice.Neoplasma 1991; 38: 49–55.
Kopper L, Magyarosy E, Nagy P, Lapis K, Szamel I, Eckhardt S, Csata S, Wabrosh G, Repassy D. Renal cell carcinoma—xenotransplantation into immuno-suppressed mice.Oncology 1984; 41: 19–24.
Rajnay J, Kopper L, Lapis K. Chemotherapeutic response of squamous cell carcinoma xenografts (subcutaneous and subrenal capsule assay.Oncology 1987; 44: 307–311.
Kopper L, Bankfalvi A, Mihalik R, Paloczi K, Benczur M, Lapis K. Phenotypic characteristics of three human non-Hodgkin lymphoma lines: flow cytometric analysis after long-term maintenance.Clin Exp Immunol 1988; 74: 295–299.
Tímár J, Kovalszky I, Paku S, Lapis K, Kopper L. Two human melanoma xenografts with different metastatic capacity and glycosaminoglycan pattern.J Cancer Res Clin Oncol 1989; 115: 554–557.
Morgan RT, Woods LK, Moore GE, Quinn LA, McGavran L, Gordon SG. Human cell line (COLO 357) of metastatic pancreatic adenocarcinoma.Int J Cancer 1980; 25: 591–598.
Meitner PA, Kajiji SM, LaPosta-Frazier N, Bogaars HA, Jolly GA, Dexter DL, Calabresi P, Turner MD. “COLO 357,” a human pancreatic adenosquamous carcinoma: growth in artificial capillary culture and in nude mice.Cancer Res 1983; 43: 5978–5985.
Yunis AA, Arimura GK, Russin DJ. Human pancreatic carcinoma (MIA PaCa-2) in continuous culture: sensitivity to asparaginase.Int J Cancer 1977; 19: 218–235.
Akagi T, Kimoto T. Establishment and characteristics of a human pancreatic cancer cell line (HCG-25).Acta Pathol Jpn 1977; 27: 51–58.
Tan MH, Nowak NJ, Loor R, Ochi H, Sandberg AA, Lopez C, Pickren JW, Berjian R, Douglass HO Jr, Chu TM. Characterization of a new primary human pancreatic tumor line.Cancer Invest 1986; 4: 15–23.
Lieber M, Mazzetta J, Nelson-Rees W, Kaplan M, Todaro G. Establishment of a continuous tumor-cell line (PANC-1) from a human carcinoma of the exocrine pancreas.Int J Cancer 1975; 15: 741–747.
Iwamura T, Katsuki T, Ide K. Establishment and characterization of a human pancreatic cancer cell line (SUIT-2) producing carcinoembryonic antigen and carbohydrate antigen 19-9.Jpn J Cancer Res 1987; 78: 54–62.
Kyriazis AP, Kyriazis AA, Scarpelli DG, Fogh J, Rao S, Lepera R. Human pancreatic adenocarcinoma line Capan-1 in tissue culture and the nude mouse.Am J Pathol 1982; 106: 250–260.
Siedlar M, Stachura J, Szczepanik A, Popiela T, Mattei M, Vendetti S, Colizzi V, Zembala M. Characterization of human pancreatic adenocarcinoma cell line with high metastatic potential in SCID mice.Invasion Metast 1995; 15: 60–69.
Dexter DL, Matook GM, Meitner PA, Bogaars HA, Jolly GA, Turner MD, Calabresi P. Establishment and characterization of two human pancreatic cancer cell lines tumorigenic in athymic mice.Cancer Res 1982; 42: 2705–2714.
Chen WH, Horoszewicz JS, Leong SS, Shimano T, Penetrante R, Sanders WH, Berjian R, Douglass HO, Martin EW, Chu TM. Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites.In Vitro 1982; 18: 24–34.
Kyriazis AA, Kyriazis AP, Sternberg CN, Sloane NH, Loveless JD. Morphological, biological, biochemical, and karyotypic characteristics of human pancreatic ductal adenocarcinoma Capan-2 in tissue culture and the nude mouse.Cancer Res 1986; 46: 5810–5815.
Okabe T, Yamaguchi N, Ohsawa N. Establishment and characterization of a carcinoembryonic antigen (CEA)-producing cell line from a human carcinoma of the exocrine pancreas.Cancer 1983; 51: 662–668.
Kallioniemi OP. Comparison of fresh and paraffin-embedded tissue as starting material for DNA flow cytometry and evaluation of intratumor heterogeneity.Cytometry 1988; 9: 164–169.
Alanen KA, Klemi PJ, Joensuu H, Kujari H, Pekkal E. Comparison of fresh, ethanol-preserved, and paraffin-embedded samples in DNA flow cytometry.Cytometry 1989; 10: 81–85.
Suto T, Sasaki K, Sugai T, Kanno S, Saito K. Heterogeneity in the nuclear DNA content of cells in carcinomas of the biliary tract and pancreas.Cancer 1993; 72: 2920–2928.
Yoshimura T, Manabe T, Suwa H, Imamura T, Wang Z, Ohshio G, Yamabe H, Matsumoto M, Ogasahara K, Takasan H. Nuclear DNA content as a prognostic predictor in carcinoma of the pancreas.Int J Pancreatol 1993; 14: 29–36.
Yoshimura T, Manabe T, Imamura T, Imanishi K, Ohshio G, Yamabe H, Kitamura O, Matsumoto M, Ogasahara K, Takasan H, Tobe T. Flow cytometric analysis of nuclear DNA content of duct cell carcinoma of the pancreas.Cancer 1992; 70: 1069–1074.
Herrera MF, van-Heerden JA, Katzmann JA, Weiland LH, Nagorney DM, Ilstrup D. Evaluation of DNA nuclear pattern as a prognostic determinant in resected pancreatic ductal adenocarcinoma.Ann Surg 1992; 215: 120–124.
Hyoty M, Visakorpi T, Kallioniemi OP, Mattila J, Laippala P, Nordback I. Prognostic value of analysis of DNA in pancreatic adenocarcinoma by flow cytometry.Eur J Surg 1991; 157: 595–600.
MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinsases to tumor progression, invasion and metastasis.Cancer Metast Rev 1995; 14: 351–362.
Gress TM, Muller-Pillasch F, Lerch MM, Friess H, Buchler M, Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer.Int J Cancer 1995; 62: 407–413.
Noel A, Emonard H, Polette M, Birembaut P, Foidart JM. Role of matrix, fibroblasts and type IV collagenases in tumor progression and invasion.Pathol Res Pract 1994; 190: 934–941.
Stearns ME, Wang M. Type IV collagenase (M(r) 72,000) expression in human prostate: benign and malignant tissue.Cancer Res 1993; 53: 878–883.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zalatnai, A., Bocsi, J., Timár, F. et al. Establishment and characterization of a new transplantable pancreatic cancer xenograft (PZX-5) in immunosuppressed mice. Int J Pancreatol 23, 51–62 (1998). https://doi.org/10.1007/BF02787503
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02787503