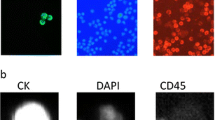
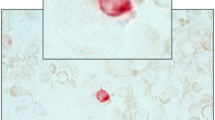
Abstract
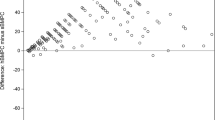
Peripheral blood stem cells were mobilised with G-CSF from steady-state haemopoiesis after previous anthracyclin-containing standard dose chemotherapy in patients with high-risk breast cancer. 48 samples were obtained from patients with stage II–III breast cancer and ≥10 lymph nodes, 15 samples from patients with chemotherapy sensitive metastatic disease, and 13 samples from women with inflammatory breast cancer. 44 samples were first or single leukaphereses and 32 samples were second or third harvests. Aliquots were searched for contaminating tumour cells by immunocytochemistry (IC) and cytokeratin-19 reverse transcriptase polymerase chain reaction (rtPCR). The median count of MNCs examined by IC was 2×106; cDNA prepared from 2×107 cells was subjected to PCR. Fifty-nine samples were examined by immunocytochemistry, 36 samples by rtPCR, and 19 samples by both techniques. Samples investigated by IC and rtPCR were judged as positive if there was at least one positive test. On the whole, 42/79 (55.3%) of the samples were positive with an insignificant trend to a higher positivity rate in second or subsequent leukaphereses (52.3% vs 59.3%). The median tumour cell load per 106 MNCs was low with 0.5(0–7) cells in all, and a total of 2.2 (0.5–7) cells in positive specimen. Differences in the cancer cell load of first and subsequent leukaphereses and between subgroups of patients were not found. PCR and IC gave consistent results in 63.2%. This phenomenon can be explained by the greater sensitivity of the molecular method and by a Poisson distribution of coharvested tumour cells in samples. Tumour cell contamination in G-CSF mobilised stem cells from patients with breast cancer from steady state haemopoiesis after preceding anthacyclin-containing chemotherapy is frequent, but the tumour cell load is low. To allow a comparison of different studies dealing with cancer cell contamination in stem cells, standardisation of assays is necessary.
Similar content being viewed by others
References
Gradishar WJ. Inflammatory breast cancer: the evolution of multimodality treatment strategies.Semin Surg Oncol 1996;12(5): 352–363.
Hortobagyi GN. Multidisciplinary management of advanced primary and metastatic breast cancer.Cancer 1994;74(1 Suppl.): 416–423.
Weidner N. Prognostic factors in breast carcinoma.Curr Opin Obstet Gynecol 1995;7(1): 4–9.
Peters WPet al. High-dose chemotherapy and autologous bone marrow support as consolidation after standard-dose adjuvant therapy for high-risk primary breast cancer.J Clin Oncol 1993;11(6): 1132–1143.
Zander ARet al. High dose mitoxantrone with thiotepa, cyclophosphamide and autologous stem cell rescue for high risk stage II and stage III breast cancer. German GABG-4/EH-93-Study.Bone Marrow Transplant 1996;18 (Suppl. 1): S24-S25.
Bezwoda WR, Seymour L, Dansey RD. High-dose chemotherapy with hematopoietic rescue as primary treatment for metastatic breast cancer: a randomized trial.J Clin Oncol 1995;13(10): 2483–2489.
Nitz Uet al. Significance of stem cell-supported high-dose chemotherapy in the treatment of gynecological malignancies: indications and current clinical trials in the Federal Republic of Germany].Zentralbl Gynakol 1997;119(5): 195–203.
Fields KK, Elfenbein GJ, Perkins JB, Moscinski LC. High dose versus standard dose chemotherapy for the treatment of breast cancer. A review of current concepts.Ann NY Acad Sci 1995;770: 288–304.
Datta YHet al. Sensitive detection of occult breast cancer by the reverse-transcriptase polymerase chain reaction.J Clin Oncol 1994;12(3): 475–482.
Brugger Wet al. Mobilization of tumor cells and hematopoietic progenitor cells into peripheral blood of patients with solid tumors.Blood 1994;83(3): 636–640.
Ross AAet al. Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques.Blood 1993;82(9): 2605–2610.
Diel IJet al. Detection of tumor cells in bone marrow of patients with primary breast cancer: a prognostic factor for distant metastasis.J Clin Oncol 1992;10(10): 1534–1539.
Schulze Ret al. Tumor cell contamination of peripheral blood stem cell transplants and bone marrow in high-risk breast cancer patients.Bone Marrow Transplant. 1997;19(12): 1223–1228.
Passos-Coelho JLet al. Absence of breast cancer cells in a single-day peripheral blood progenitor cell collection after priming with cyclophosphamide and granulocyte-macrophage colony-stimulating factor.Blood 1995;85(4): 1138–1143.
Passos-Coelho JLet al. Similar breast cancer cell contamination of single-day peripheral-blood progenitor-cell collections obtained after priming with hematopoietic growth factor alone or after cyclophosphamide followed by growth factor.J Clin Oncol 1996:14(9): 2569–2575.
Schlimok Get al Epithelial tumor cells in bone marrow of patients with colorectal cancer: immunocytochemical detection, phenotypic characterization, and prognostic significance.J Clin Oncol 1990;8(5): 831–837.
Krüger Wet al. Reverse transcriptase/polymerase chain reaction detection of cytokeratin-19 mRNA in bone marrow and blood of breast cancer patients.J Cancer Res Clin Oncol 1996;122(11): 679–686.
Joshi SS, Jackson JD, Sharp JG. Comparison of the growth and metastasis of four human intestinal tumor cell line xenografts.Tumour Biol 1989;10(3): 117–125.
Sharp JG. Micrometastases and transplantation.J Hematother 1996;5(5): 519–524.
Traystman MDet al. Comparison of molecular cytokeratin 19 reverse transcriptase polymerase chain reaction (CK19 RT-PCR) and immunocytochemical detection of micrometastatic breast cancer cells in hematopoietic harvests.J Hematother 1997;6(6): 551–561.
Jung Ret al. Specificity of reverse transcriptase polymerase chain reaction assays designed for the detection of circulating cancer cells is influenced by cytokines in vivo and in vitro.Brit J Cancer 1998;78(9): 1194–1198.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krüger, W., Tögel, F., Kröger, N. et al. Tumour cell detection in G-CSF mobilised stem cell harvests of patients with breast cancer. Med Oncol 16, 17–22 (1999). https://doi.org/10.1007/BF02787354
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02787354