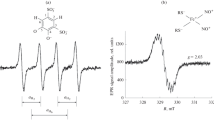
Abstract
Trace elements play an important role in oxygen metabolism and therefore in the formation of free radicals. Whereas iron and copper are usually the main enhancers of free radical formation, other trace elements, such as zinc and selenium, protect against the harmful effects of these radicals.
To investigate the different protective mechanisms of zinc on radical formation, we examined the effects of added zinc and copper on superoxide dismutase activity. We also studied the effects of copper and iron on xanthine oxidase activity and on the Haber-Weiss cycle (iron, superoxide, and hydrogen peroxide), which generates hydroxyl radicals in vitro. The hypoxanthine/xanthine oxidase radical generating system contained a variety of different physiological ligands for binding the iron.
This study confirmed the inhibitory effect of copper on xanthine oxidase activity. Moreover, it demonstrated that zinc inhibited hydroxyl radical formation when this formation was catalyzed by a citrate-iron complex in the hypoxanthine/xanthine oxidase reaction. Finally, human blood plasma inhibited citrate-iron-dependent hydroxyl radical formation under the same conditions. Although trace elements seemed responsible for this antioxidant activity of plasma, it is likely that zinc played no role as a plasma antioxidant. Indeed, calcium appeared to be responsible for most of this effect under our experimental conditions.
Similar content being viewed by others
Abbreviations
- ADP:
-
Adenosine diphosphate
- EDTA:
-
Ethylene-diaminetetraacetic acid tetrasodium salt
- HX:
-
Hypoxanthine sodium salt
- KMB:
-
2-Keto-4-thiomethyl butyric acid
- oOH:
-
Hydroxyl radical
- SOD:
-
Native superoxide dismutase
- SODCu2E2:
-
Zinc-depleted superoxide dismutase
- SODE2E2:
-
Zinc- and copper-depleted superoxide dismutase
- TRIS:
-
Tris(hydroxyethyl)aminomethane
- XO:
-
Xanthine oxidase
References
J. M. C. Gutteridge,Biochim. Biophys. Acta. 834, 144–148 (1985).
J. M. C. Gutteridge,FEBS Lett. 128, 343–346 (1981).
G. J. Quilan, C. Coudray, A. Hubbard, and J. M. C. Gutteridge,J. Pharm. Sci. 81, 611–614 (1992).
M. Fontecave and J. L. Pierre,BioMetals 4, 144,145 (1991).
M. S. Baker and J. M. Gebicki,Arch. Biochem. Biophys. 246, 581–588 (1986).
R. A. Floyd and C. Ann Lewis,Biochemistry 22, 2645–1649 (1983).
C. F. Babbs and M. G. Steiner,Meth. Enzymol. 186, 137–147 (1990).
C. Beauchamp and I. Fridovich,J. Biol. Chem. 245, 4641–4646 (1970).
J. DiGuiseppi and I. Fridovich,Arch. Biochem. Biophys. 205, 323–329 (1980).
H. C. Sutton,Free Rad. Biol. Med. 1, 195–202 (1985).
R. Richmond, B. Halliwell, J. Chauhan, and A. Darbre,Anal. Biochem. 118, 328–335 (1981).
S. Singh and R. C. Hider,Anal. Biochem. 171, 47–54 (1988).
G. R. Buettner, L. W. Oberley, and S. W. H. Chan Leuthauser,Photochem. Photobiol. 28, 693–695 (1978).
F. Boucher, S. Pucheu, C. Coudray, A. Favier, and J. de Lerris,FEBS Lett. 302, 261–264 (1992).
B. Halliwell and J. M. C. Gutteridge,Biochem. J. 219, 1–14 (1984).
J. Lunec, D. G. Wickens, T. L. Graff, and T. L. Dormandy, inInflamatory Diseases and Copper, Ed. John R. J. Sorenson, The Humana Press Inc., Totowa, NJ, 1982.
C. Coudray, M. J. Richard, F. Laporte, P. Faure, A. M. Roussel, and A. Favier,J. Nutr. Med. 3, 13–26 (1992).
K. Takahash, P. E. Newburger, and H. J. Cohen,J. Clin. Invest. 77, 1402–1404 (1986).
C. Coudray, F. Boucher, M. J. Richard, J. de Leiris, and A. Favier,Biol. Trace Elem. Res. 30, 103–118 (1991).
H. Korpela, A. Kauppila, U. M. Makila, H. Sundstrom, L. Viinikka, and E. Yrjanheikki,Life Chem. Rep. 3, 203–206 (1985).
J. M. C. Gutteridge, C. Hill, and D. R. Blake,Clin. Chim. Acta 139, 85–90 (1984).
M. Chvapil, J. N. Ryan, and C. F. Zukoski,Proc. Soc. Exp. Biol. Med. 141, 150–153 (1972).
M. Chvapil, J. N. Ryan, and C. F. Zukoski,Proc. Soc. Exp. Biol. Med. 140, 642–646 (1972).
M. Chvapil, I. Sipes, J. C. Ludwing, and S. C. Halladay,Biochem. Pharmacol. 24, 917–919 (1975).
M. Chvapil, L. Stankova, C. Zukoski IV, and C. Zukoski III,J. Lab. Clin. Med. 89, 135–146 (1977).
J. F. Sullivan, M. N. Jetton, H. K. J. Hahn, and R. E. Burch,Am. J. Clin. Nutr. 33, 51–56 (1980).
H. Anttinen, L. Ryhnen, U. Puistola, A. Arranto, and A. Oikainen,Gastroenterology 86, 532–539 (1984).
D. E. Coppen, D. E. Richardson, and R. J. Cousins, (Abstract) inFood Science and Human Nutrition, Chemistry Department, University of Florida, Gainesville, 1985.
S. Kubov, t. M. Bray, and W. J. Bettger,J. Nutr. 116, 1054–1060 (1986).
S. Ishimitsu, S. Fujimoto, and A. Ohara,Chem. Pharm. Bull. 32, 4645–4649 (1984).
S. Marklund and G. Marklund,Eur. J. Biochem. Biophys. 205, 323–329 (1980).
S. J. Weiss, P. K. Rustagi, and A. F. LoBuglio,J. Exp. Med. 147, 316–323 (1978).
N. Koukay, M. J. Richard, and A. Favier, inBiologie Prospective, Comptes rendus du 7 e colloque de Pont-a-Mousson. M. M. Galteau, G. Siest, and J. Henny, John Libbey Eurotext, Paris, 1989, pp. 545–548.
N. Koukay, Rapport de D.E.A de biologie moléculaire et cellulaire. Présenté à l'Université Scientifique et Médicale de Grenoble, France (1985).
J. M. McCord and I. Fridovich,J. Biol. Chem. 244, 6049–6055 (1969).
F. Haber and J. Weiss,Proc. R. Soc. Lett. 9, 775–779 (1934).
B. Halliwell,FEBS Lett. 96, 238–242 (1987).
U. V. Weser, G. Barth, C. Djerassi, H. J. Hartmann, P. Krauss, G. Voelcker W. Voelter, and W. Voetsch,Biochem. Biophys. Acta 278, 28–44 (1972).
B. Halliwell,FEBS Lett. 92, 321–326 (1978).
J. M. McCord and E. D. Day,FEBS Lett. 86, 139–142 (1978).
J. M. C. Gutteridge,Free Rad. Biol. Med. 11, 401–406 (1991).
M. C. Grootveld, J. D. Bell, B. Halliwell, O. I. Aruoma, H. Bombord, and P. J. Sadler,J. Biol. Chem. 264, 4417–4422 (1989).
H. G. Parkes, R. E. Allen, A. Fwst, D. R. Blake, and M. C. Grootveld,J. Pharm. Biomed. Anal. 9, 29–32 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Coudray, C., Rachidi, S. & Favier, A. Effect of zinc on superoxide-dependent hydroxyl radical production in vitro. Biol Trace Elem Res 38, 273–287 (1993). https://doi.org/10.1007/BF02785311
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02785311