Abstract
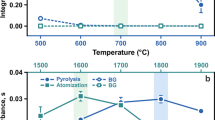
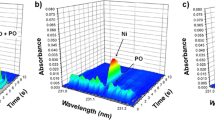
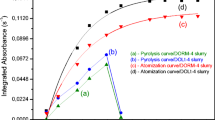
Graphite furnace atomic absorption spectrophotometry is a method used for the measurement of low concentrations of manganese (ppb range). Despite the widespread use of this technique, there is considerable inconsistency concerning sample preparation and choice of instrumental parameters. In this paper, we determined manganese concentrations of National Bureau of Standards (NBS) bovine liver by both graphite furnace (Instrumentation Laboratory IL 555B) and flame atomic absorption following wet digestion of the sample with nitric acid. The following instrumental parameters for the graphite furnace were found optimal for the measurement of manganese in digested NBS bovine liver: inert gas flow=14 SCFH, drying temperature 100°C/15 s (step 1), 125°C/15 s (step 2), pyrolysis temperature 500°C/15 s (step 3), and 1000°C/15 s (step 4); atomization temperature 2250°C/10 s (step 5). For optimal results, the nitric acid concentration of the sample should be between 2 and 4M. There were no significant differences found for manganese concentrations determined by either peak height or peak area measurement. Additionally, no significant differences were found in manganese concentrations determined by flame or furnace methods. Assuming proper sample preparation and choice of instrumental parameters, values obtained for manganese concentration by graphite furnace and flame atomic absorption spectrophotometry are similar. Therefore, data obtained by these two methods can be compared directly.
Similar content being viewed by others
References
J. Versieck, R. Cornelis, G. Lemey, and A. J. De Rudder,Clin. Chem. 26, 531 (1980).
P. S. Papavasiliou and G. Cotzias,J. Biol. Chem. 236, 2365 (1961).
M. S. Clegg, C. L. Keen, B. Lonnerdal, and L. S. Hurley,Biol. Trace Element Res. 3, 107 (1981).
M. S. Clegg, C. L. Keen, B. Lonnerdal, and L. S. Hurley,Biol. Trace Element Res. 3, 237 (1981).
R. E. Sturgeon, C. L. Chakraborti, I. S. Maines, and P. C. Bertels,Anal. Chem. 47, 1240 (1975).
R. E. Sturgeon, C. L. Chakraborti, and P. C. Bertels,Anal. Chem. 47, 1251 (1975).
A. Smeyers-Verbeke, Y. Micholfe, P. Vandenwinkel, and P. C. Mussart,Anal. Chem. 48, 124 (1976).
P. Geladi and F. Adams,Anal. Chim. Acta 105, 219 (1979).
D. L. D’Amico and M. L. Klawans,Anal. Chem. 48, 1469 (1976).
E. Bonilla,Clin. Chem. 24, 471 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clegg, M.S., Keen, C.L., Lonnerdal, B. et al. Analysis of trace elements in animal tissues. Biol Trace Elem Res 4, 145–156 (1982). https://doi.org/10.1007/BF02783254
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02783254
Index Entries
- Manganese determination, by graphite furnace AAS
- atomic absorption spectrophotometry, of Mn in animal tissues
- biological samples
- biotrace element analysis, of Mn
- analysis of biological trace elements
- instrumental parameters, in biological trace element analysis
- methods in biological trace element analysis