Abstract
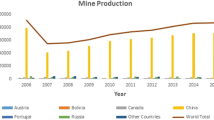
For a natural substance that is mined and for which the world total production is in excess of 3 million tons, it would not be surprising to discover there is considerable exposure to boron (B) and its salts. Human exposure can arise from a variety of natural sources, such as soil and water, and artificially in soils via fertilizer and in waters via discharges through its use in household products and cosmetics, or via its use in pesticides, preservatives, and pharmaceuticals. Receiving waters can also obtain B through industrial usage.
Indirect and probably insignificant consumer exposure can arise via the use of borates in glass products, flame retardants, and enamels, these applications comprising the major proportion of borate usage. The general intake of B via its presence in food and water is around 1–7 mg/d, but the range of intake values is large and depends on geographical region, dietary habits (with nuts, fresh and dried fruits, and wine providing particularly rich sources), as well as the method of analysis employed. Drinking waters typically contain <1 mg B/L, but the range is large, and some populations will be exposed to considerably more than 1 mg B/L. B via food and water represents the greatest source of exposure for the general consumer.
Sodium borate and boric acid are widely used in consumer products, and perborate is used in detergents, but even though there is a high potential for exposure, the internal dose of B to consumers via its use in household and personal products is very low partly owing to insignificant amounts of dermal absorption across normal skin.
Similar content being viewed by others
References
W. G. Wood, An introduction to boron; history, sources, use and chemistry,Env. Health Perspect. 102, Suppl.7, 5–11 (1994).
P. A. Lyday, cited in ECETOC 1995 (1992).
C. D. Hunt, Concentration of boron and other elements in human foods and personal care products,J. Am. Diet Assoc. 91(5), 558–568 (1991).
C. J. Rainey, R. E. Christensen, L. A. Nyquist, P. L. Strong, and J. R. Coughlin, The dietary consumption of boron in the USA, Abstract 4536,FASEB J. 10, No. 3, A, 785 (1997).
ECETOC, Reproductive and general toxicology of some inorganic borates and risk assessment for human being, Technical report 63 (1995).
R. D. Barr, W. B. Clarke, R. M. Clarke, J. Venturelli, G. R. Norman, and R. G. Downing, Regulation of lithium and boron levels in normal human blood, environmental and genetic considerations,J. Lab. Clin. Med. 12(4), 614–619 (1993).
H. E. Allen, M. A. Halley-Henderson, and C. N. Hass, Chemical composition of bottled mineral water,Arch. Env. Health 44(2), 102–116 (1989).
B. D. Culver, R. G. Smith, R. J. Brotherton, P. L. Strong, and T. M. Gray, Boron, inPatty’s Industrial Hygiene and Toxicology, 4th ed., Chapter 42 (1994). Wiley, New York, 4411–4448.
R. B. Goldbloom and A. Goldbloom, Boric acid poisoning report of four cases and a review of 109 cases from the world literature.J. Pediat. 43, 631–643.
T. L. Litovitz, W. Klein, G. M. Oderda, and B. F. Schmitz, Clinical manifestations of toxicity in a series of 784 boric acid ingestions,Am. J. Emerg. Med. 6(3), 209–213 (1988).
CIR, Final report on the safety assessment of sodium borate and boric acid,J. Am. College Toxicol. 2(7), 87–125 (1983).
G. Stuttgen, T. Siebel, and B. Aggerbeck, Absorption of boric acid through human skin depending on the type of vehicle,Arch. Dermatol Res. 272, 21–29 (1982).
Borax Research, personal communication (1997).