Summary
It is now relatively well established that chenodeoxycholic acid (CDCA) and ursodeoxycholic acid (UDCA), which are 7-OH epimer, are cholesterol gallstone dissolving agent.
We investigated physical-chemical properties of CDCA and UDCA with respect to critical micellar concentration (CMC), micellar molecular weight (MMW) and solubilizing power of cholesterol.
CMCs of CDCA and UDCA, measured by surface tension method, were 3.6 mM and 6.4 mM respectively.
Light scattering technique revealed the MMW as 7700 in CDCA and 7400 in UDCA of only one molecule’s difference per micelle in the aggregation number.
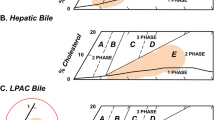
Solubilization of cholesterol in model bile system disclosed remarkable difference. The degree of solubilized cholesterol by UDCA was smaller than that of CDCA by a factor of about 19. Addition of lecithin produced a greater increment of solubilized cholesterol in UDCA, but the solubilizing power was still larger in CDCA.
It is curious and of interest that there exists much difference in the aspects of solubilizing efficiency for cholesterol in thein vitro model system, inspite of their little difference in the molecular structure, CMC and MMW between CDCA and UDCA. 7Β-OH might affect the function of micelle against solubilization in UDCA.
Similar content being viewed by others
References
Danzinger RG, Hofmann AF, Schoenfield LJ, Thistle JL: Dissolution of cholesterol gallstone by chenodeoxycholic acid. New Eng J Med 286: 1–8, 1972
Hofmann AF: Desaturation of bile and cholesterol gallstone dissolution with chenodeoxycholic acid. Am J Clin Nut 30: 993–1000, 1977
Thistle JL, Hofmann AF, Ott BJ, Stephens DH: Chenotherapy for gallstone dissolution: 1. Efficacy and safety. JAMA 239: 1041–1046, 1978
Makino I., Shinozaki K., Yoshino K., Nakagawa S.: Dissolution of cholesterol gallstones by ursodeoxycholic acid. Jpn J Gastroenterol 72: 690–701, 1975
Nair PP, Kritchevsky D.: The Bile Acids; vol. 1. Chemistry. Plenum Press, New York, 1971
Hanahan DJ, Turner MB, Jayko ME: The isolation of egg phosphatidyl choline by an adsorption column technique. J Biol Chem 192: 623–628, 1951
Glasstone S., Lewis D.: Elements of Physical Chemistry. Maruzen, Tokyo, 1961
Debye P.: Molecular-weight determination by light scattering. J Phys Chem 51: 18–24, 1947
Stacey KA: Light Scattering in Physical Chemistry. Butterworths, London, 1956
Furusawa T., Nakama T., Hisadome T., Itoh H.: Basic studies on cholesterol solubilization in bile salt solution. Gastroenterol Jpn 11: 356–362, 1976
Furusawa T., Nakama T., Itoh H., Hisadome T.: Reappraisal of cholesterol solubilization in bile salt-lecithin solution and the stability of bile, ibid Gastroenterol Jpn 12: 253–262, 1977
Sperry WM, Webb M.: A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem 187: 97–106, 1950
Miles GD: Minima in surface tension and interfacial tension curves. J Phys Chem 49: 71–75, 1945
Fedorowski T., Salen G., Zaki FG, Shefer S., Mosbach EH: Comparative effects of ursodeoxycholic acid and chenodeoxycholic acid in the rhesus monkey; biochemical and ultrastructural studies. Gastroenterology 74: 75–81, 1978
Maton PN, Murphy GM, Dowling RH: Ursodeoxycholic acid treatment of gallstones; dose-response study and possible mechanism of action. Lancet 2: 1297–1301, 1977
Stiel A., Czygan P., Kommerell B., Weis HJ, Holter-müller KH: Ursodeoxycholic acid versus chenodeoxycholic acid; comparison of their effects on bile acid and bile lipid composition in patients with cholesterol gallstones. Gastroenterology 75: 1016–1020, 1978
Carey MC, Small DM: Micelle formation by bile salts; physical-chemical and thermodynamic considerations. Arch Intern Med 130: 506–527, 1972
Hisadome T., Nakama T., Itoh H., Furusawa T.: Surface tension measurement of bile salt solutions with automatic-electro-surface-balance. J Jpn Med Soc Biol Interface 8: 115–117, 1977
Small DM: Size and structure of bile salt micelles. Advances Chem Ser 84: 31–52, 1968
Oakenfull DG, Fisher LR: The role of hydrogen bonding in the formation of bile salt micelles. J Phys Chem 81: 1838–1841, 1977
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hisadome, T., Nakama, T., Itoh, H. et al. Physical-chemical properties of chenodeoxycholic acid and ursodeoxycholic acid. Gastroenterol Jpn 15, 257–263 (1980). https://doi.org/10.1007/BF02774276
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02774276