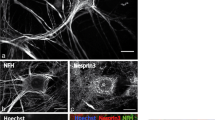
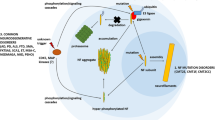
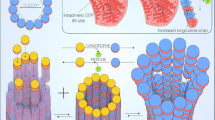
Abstract
The prominent death of central neurons in Alzheimer's and Parkinson's is reflected by changes in cell shape and by the formation of characteristic cytoskeletal inclusions (neurofibrillary tangles, Lewy bodies). This review focuses on the biology of neurofilaments and microtubule-associated proteins and identifies changes that can occur to these elements from basic and clinical research perspectives. Attention is directed at certain advances in neurobiology that have been especially integral to the identification of epitope domains, protein isoforms, and posttranslational (phosphorylation) events related to the composition, development, and structure of the common cytoskeletal modifications.
Recently, a number of experimental strategies have emerged to simulate the aberrant changes in neurodegenerative disorders and gain insight into possible molecular events that contribute to alterations of the cytoskeleton. Descriptions of specific systems used to induce modifications are presented. In particular, unique neural transplantation methods in animals have been used to probe possible molecular and cellular conditions concerned with abnormal cytoskeletal changes in neurons.
Similar content being viewed by others
References
Aletta J. M., Lewis S. A., Cowan N. J., and Greene L. A. (1988) Nerve growth factor regulates both the phosphorylation and steady-state levels of microtubule-associated protein 1.2 (MAP 1.2).J. Cell Biol. 106, 1573–1581.
Alzheimer A. (1907) Über eine eigenartige Erkrankung der Hirnrinde.Algemeine Zeitschrift für Psychiatrie 64, 146–148.
Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., and Kahn J. (1982) Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants.Nature 298, 84–86.
Arai H., Lee V. M.-Y., Otvos L., Greenberg B. D., Lowery D. E., Sharma S. K., Schmidt M. L., and Trojanowski J. Q. (1990) Defined neurofilament, and β-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaques.Proc. Natl. Acad. Sci. USA 87, 2249–2253.
Arai H., Lee V. M.-Y., Hill W. D., Greenberg B. D., and Trojanowski J. Q. (1992a) Lewy bodies contain beta-amyloid precursor proteins of Alzheimer's disease.Brain Res. 585, 386–390.
Arai H., Schmidt M. L., Lee V. M.-Y., Hurtig H. I., Greenberg B. D., Adler C. H., and Trojanowski J. Q. (1992b) Epitope analysis of senile plaque components in the hippocampus of patients with Parkinson's disease.Neurology 42, 1315–1322.
Baas P. W., Deitch J. S., Black M. M., and Banker G. A. (1988) Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite.Proc. Natl. Acad. Sci. USA 85, 8335–8339.
Baas P. W. and Black M. M. (1990) Individual microtubules in the axon consist of domains that differ in both composition and stability.J. Cell Biol. 111, 495–509.
Baas P. W., Pienkowski T. P., and Kosik K. S. (1991) Processes induced by tau expression in Sf9 cells have an axon-like microtubule organization.J. Cell Biol. 115, 1333–1344.
Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., and Wisniewski H. M. (1989a) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease.Brain Res. 477, 90–99.
Bancher C., Lassmann H., Budka H., Jellinger D., Grundke-Iqbal I., Wiche G., Seitelberger F., and Wisniewski H. M. (1989b) An antigenic profile of Lewy bodies: immunocytochemical indication for protein phosphorylation and ubiquitination.J. Neuropathol. Exp. Neurol. 48, 81–93.
Bernhardt R. and Matus A. (1984) Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons.J. Comp. Neurol. 226, 203–221.
Biernat J., Mandelkow E. M., Schröter C., Lichtenberg-Kraag B., Steiner B., Berling B., Meyer H., Mercken M., Vandermeeren A., Goedert M., and Mandelkow E. (1992) The switch of tau protein to an Alzheimer-like state induces the phosphorylation of two serine-proline motifs upstream of the microtubule binding region.EMBO J. 11, 1593–1597.
Bigot D. and Hunt S. P. (1991) The effects of quisqualate and nocodazole on the organization of MAP2 and neurofilaments in spinal cord neurons in vitro.Neurosci. Lett. 131, 21–26.
Binder L. I., Frankfurter A., Kim H., Caceres A., Payne M. R., and Rebhun L. I. (1984) Heterogeneity of microtubule-associated protein 2 during rat brain development.Proc. Natl. Acad. Sci. USA 81, 5613–5617.
Binder L. I., Frankfurter A., and Rebhun L. I. (1985) The distribution of tau in the mammalian nervous system.J. Cell Biol. 101, 1371–1378.
Bizzi A., Crane R. C., Autilio-Gambetti L., and Gambetti P. (1984) Aluminum effect of slow axonal transport: a novel impairment of neurofilament transport.J. Neurosci. 4, 722–731.
Bizzi A. and Gambetti P. (1986) Phosphorylation of neurofilaments is altered by aluminum intoxication.Acta Neuropathol. 71, 154–158.
Black M. M., Baas P. W., and Humphries S. (1989) Dynamics of α-tubulin deacetylation in intact neurons.J. Neurosci. 9, 358–368.
Bloom G. S., Schoenfeld T. A., and Vallee R. B. (1984) Widespread distribution of the major polypeptide component of MAP1 (microtubule-associated protein 1) in the nervous system.J. Cell Biol. 98, 320–330.
Bondareff W., Wischik C. M., Novak M., Amos W. B., Klug A., and Roth M. (1990) Molecular analysis of neurofibrillary degeneration in Alzheimer's disease: an immunohistochemical study.Am. J. Pathol. 137, 711–723.
Braak H., Braak E., Grundke-Iqbal I., and Iqbal K. (1986) Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques.Neurosci. Lett. 65, 351–355.
Braak H. and Braak E. (1988) Neuropil threads occur in dendrites of tangle-bearing nerve cells.Neuropathol. Appl. Neurobiol. 14, 39–44.
Bramblett G. T., Trojanowski J. Q., and Lee V. M.-Y. (1992) Regions with abundant neurofibrillary pathology in human brain exhibit a selective reduction in levels of binding-competent tau and accumulations of abnormal tau-isoforms (A68 proteins).Lab. Invest. 66, 212–222.
Bramblett G. T., Goedert M., Jakes R., Merrick S. E., Trojanowski J. Q., and Lee V. M-Y. (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding.Neuron 10, 1089–1099.
Brion J. P., Guilleminot J., Couchie D., Flament Durand J., and Nunez J. (1988) Both adult and juvenile tau microtubule-associated proteins are axon specific in the developing and adult rat cerebellum.Neurosci. 25, 139–146.
Brion J. P., Hanger D. P., Couck A. M., and Anderton B. (1991) A68 proteins in Alzheimer's disease are composed of several tau isoforms in a phos phorylated state which affects their electrophoretic mobilities.Biochem. J. 279, 831–836.
Brugg B. and Matus A. (1991) Phosphorylation determines the binding of microtubule-associated protein 2 (MAP-2) to microtubules in living cells.J. Cell Biol. 114, 735–743.
Burgoyne R. D. and Cuming R. (1984) Ontogeny of microtubule-associated protein 2 in rat cerebellum: differential expression of the doublet polypeptides.Neuroscience 11, 157–167.
Burgoyne R. D. (1991) High molecular weight microtubule-associated proteins of brain, inThe Neuronal Cytoskeleton (Burgoyne R. D., ed.), Wiley Liss, New York, pp. 75–91.
Buxbaum J. D., Gandy S. E., Cicchetti P., Ehrlich M. E., Czernik A. J., Fracasso P. R., Ramabhadran T. V., Unterbeck A. J., and Greengard P. (1990) Processing of Alzheimer β/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation.Proc. Natl. Acad. Sci. USA 87, 6003–6006.
Caceres A. Kosik K. S. (1990) Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons.Nature 343, 461–463.
Calvert R. A. and Anderton B. H. (1985) A microtubulek-associated protein (MAP1) which is expressed at elevated levels during development in rat cerebellum.EMBO J. 4, 1171–1176.
Carden M. J., Schlaepfer W. W., and Lee, V. M.-Y. (1985) The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state.J. Biol. Chem. 260, 9805–9817.
Chauhan N. B., Spencer P. S., and Sabri M. I. (1993) Acrylamide-induced depletion of microtubule-associated proteins (MAP1 and MAP2) in the rat expyramidal system.Brain Res. 602, 111–118.
Chen, J., Kanai Y., Cowan N. J., and Hirokawa N. (1992) Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons.Nature 360, 674–677.
Chiu F.-C., Barnes E. A., Das K., Haley J., Socolow P., Macaluso F. P., and Fant J. (1989) Characterization of a novel 66 kd subunit of mammalian neurofilaments.Neuron 2, 1435–1445.
Cleveland D. W., Hwo S.-Y., and Kirschner M. W. (1977) Physical and chemical properties of purified tau factor and the role of microtubule assembly.J. Mol. Biol. 116, 227–247.
Cole G. M., Masliah E., Shelton E. R., Chan H. W., Terry R. D., and Saitoh T. (1991) Accumulation of N-terminal sequence but not the C-terminal sequence of β-protein precursor in the neuritic component of Alzheimer disease senile plaque.Neurobiol. Aging 12, 85–91.
Cork L. C., Sternberger N. H., Sternberger L. A., Casanova M. F., Struble R. G., and Price D. L. (1986) Phosphorylated neurofilament antigens in neurofibrillary tangles in Alzheimer's disease.J. Neuropathol. Exp. Neurol. 45, 56–64.
Cras P., Kawai M., Lowery D., Gonzalez-DeWhitt P., Greenberg B., and Perry G. (1991) Senile plaque neurites in Alzheimer disease accumulate amyloid precursor protein.Proc. Natl. Acad. Sci. USA 88, 7552–7556.
Davies P. (1992) Alz 50 as a reagent to assess animal models of Alzheimer's disease.Neurobiol. Aging 13, 613, 614.
DeCamilli P., Miller P. E., Navone F., Theurkauf W. E., and Vallee R. B. (1984) Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence.Neuroscience 11, 819–846.
Diaz-Nido J., Serrano L., Mendez E., and Avila J. (1988) A casein kinase II-related activity is involved in phosphorylation of microtubule-associated protein MAP 1B during neuroblastoma cell differentiation.J. Cell Biol. 106, 2057–2065.
Dickson, D. W., Davies P., Mayeux R., Crystal H., Horoupian D. S., Thompson A., and Goldman J. E. (1987) Diffuse Lewy body disease: neuropathological and biochemical studies of six patients.Acta Neuropathol. 75, 8–15.
Dickson D. W., Crystal H., Mattiace L. A., Kress Y., Schwagerl A., Ksiezak-Reding H., Davies P., and Yen S.-H. C. (1989) Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques.Acta Neuropathol. 78, 572–584.
Dickson D. W., Ksiezak-Reding H., Liu W.-K., Davies P., Crowe A., Yen, S.-H. C. (1992) Immunocytochemistry of neurofibrillary tangles with antibodies to subregions of tau protein: identification of hidden and cleaved tau epitopes and a new phosphorylation site.Acta Neuropathol. 84, 596–605.
DiPatre P. L. and Butcher L. L. (1991) Cholinergic fiber perturbations and neuritic outgrowth produced by intrafimbrial infusion of the neurofilament-disrupting agent 2,5-hexanedione.Brain Res. 539, 126–132.
Doering L. C. and Aguayo A. J. (1987) Hirano bodies and other cytoskeletal abnormalities develop in fetal rat CNS grafts isolated for long periods in peripheral nerve.Brain Res. 401, 178–184.
Doering L. C., Nilsson O. G., and Aguayo A. J. (1991a) Abnormal perikaryal immunoreactivity to the phosphorylated heavy neurofilament unit in intracerebral basal forebrain transplants.Exp. Neurol. 111, 1–8.
Doering L. C., Eriksdotter-Nilsson M., and Olson L. (1991b) Spatial distributions of cytoskeletal proteins and the NGF-receptor in septal transplants in oculo: protection from abnormal immunoreactivity by hippocampal co-grafts.Neuroscience 44, 381–392.
Doering L. C. (1992) Appropriate target interactions prevent abnormal cytoskeletal changes in neurons: A study with intra-sciatic grafts of the septum and the hippocampus.J. Neurosci. 12, 3399–3413.
Dräger U. C. and Hofbauer A. (1984) Antibodies to heavy neurofilament subunit detect a subpopulation of damaged ganglion cells in retina.Nature 309, 624–626.
Endoh R., Ogawara M., Iwatsubo T., and Mori H. (1993) Lack of the carboxy terminal sequence of tau in ghost tangles.Brain Res. 601, 164–172.
Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf, T., McClure D., and Ward P. J. (1990) Cleavage of amyloid β peptide during constitutive processing of its precursor.Science 248, 1122–1124.
Ferreira A., Busciglio J., and Caceres A. (1989) Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and tau.Dev. Brain Res. 49, 215–228.
Fine A., Ault B., Rapoport S. I. (1991) Mouse trisomy 16 neurons, a model of human trisomy 21 (Down syndrome), can be maintained by intracerebral transplantation.Neurosci. Lett. 122, 4–8.
Flament S., Delacourte A., and Mann D. H. A. (1990a) Phosphorylation of tau proteins: a major event during the process of neurofibrillary degeneration. A comparative study between Alzheimer's disease and Down's Syndrome.Brain Res. 516, 15–19.
Flament S., Delacourte A., Delaère P., Duyckaerts C., and Hauw J.-J. (1990b) Correlation between microscopical changes and Tau 64 and 69 biochemical detection in senile dementia of the Alzheimer type.Acta Neuropathol. 80, 212–215.
Forno L. S., Sternberger L. A., Sternberger N. H., Strefling A. M., Swanson K., and Eng L. F. (1986) Reaction of Lewy bodies with antibodies to phosphorylated and non-phosphorylated neurofilaments.Neurosci. Lett. 64, 253–258.
Galloway P. G., Grundke-Iqbal I., Iqbal K., and Perry G. (1988) Lewy bodies contain epitopes both shared and distinct from Alzheimer neurofibrillary tangles.J. Neuropathol. Exp. Neurol. 47, 654–663.
Galloway P. G., Mulvihill, P., and Perry G. (1992) Filaments of lewy bodies contain insoluable cytoskeletal elements.Am. J. Pathol. 140, 809–822.
Gandy S. E., Czernik A., and Greengard P. (1988) Phosphorylation of Alzheimer amyloid precursor protein peptide by protein kinase C and Ca2+/ calmodulin-dependent protein kinase II.Proc. Natl. Acad. Sci. USA 85, 6218–6221.
Garner C. C. and Matus A. (1988) Different forms of microtubule-associated protein 2 (MAP2) are encoded by separate mRNA transcripts.J. Cell Biol. 106, 779–784.
Garner C. C., Brugg B., and Matus A. (1988a) A 70 kDa microtubule-associated protein (MAP2c), related to MAP2.J. Neurochem. 50, 609–615.
Garner C. C., Tucker R. P., and Matus A. (1988b) Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites.Nature 336, 674–677.
Garner C. C., Garner A., Huber G., Kozak C., and Matus A. (1990) Molecular cloning of microtubule-associated protein 1 (MAP1A) and microtubule-associated protein 5 (MAP1B): identification of distinct genes and differential expression in developing brain.J. Neurochem. 55, 146–154.
Geisler N., Kaufmann E., Fischer S., Plessmann U., and Weber K. (1983) Neurofilament architecture combines structural principles of intermediate filaments with carboxy-terminal extensions increasing in size between triplet proteins.EMBO J. 8, 1295–1302.
Geisler N., Fischer S., Vandekerckhove J., Van Damme J., Plessmann U., and Weber K. (1985) Proteinchemical characterization of NF-H, the largest mammalian neurofilament component; intermediate filament-type sequences followed by a unique carboxy-terminal extension.EMBO J. 4, 57–63.
Geisler N., Vanderkerckhove J., and Weber K. (1987) Localization and sequence characterization of the major phosphorylation sites of the high molecular mass neurofilament proteins M and H.FEBS Lett. 221, 403–407.
Georgieff I. S., Liem R. K. M., Mellado W., Nunez J., and Shelanski M. L. (1991) High molecular weight tau: preferential localization in the peripheral nervous system.J. Cell Sci. 100, 55–60.
Gilbert M. R., Harding B. L., Hoffman P. N., Griffin J. W., Price D. L., and Troncoso J. C. (1992) Aluminum-induced neurofilamentous changes in cultured rat dorsal root ganglia explants.J. Neurosci. 5, 1763–1771.
Goedert M., Spillantini M., Jakes R., Rutherford D., and Crowther R. A. (1989) Multiple isoforms of human microtubule-associated protein-tau: sequences and localization in neurofibrillary tangles of Alzheimer's-disease.Neuron 3, 519–526.
Goedert M. and Jakes R. (1990) Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization.EMBO J. 9, 4225–4230.
Goedert M., Crowther R. A., and Garner C. C. (1991) Molecular characterization of microtubule-associated proteins tau and MAP2.Trend. Neurosci. 14, 193–199.
Goedert M., Spillantini M. G., Cairns N. J., and Crowther R. A. (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms.Neuron 8, 159–168.
Gold B. G., Griffin J. W., and Price D. L. (1985) Slow axonal transport in acrylamide neuropathy: different abnormalities produced by single-dose and continuous administration.J. Neurosci. 5 1755–1768.
Gold B. G., Price D. L., Griffin J. W., Rosenfeld J., Hoffman P. N., Sternberger N. H., and Sternberger L. A. (1988) Neurofilament antigens in acrylamide neuropathy.J. Neuropathol. Exp. Neurol. 47, 145–157
Gold B. G. and Austin D. R. (1991) Regulation of aberrant neurofilament phosphorylation in neuronal perikarya. III. Alterations following single and continuous β,β′-iminodipropionitrile administrations.Brain Res. 563, 151–162.
Golde T. E., Estus S., Usiak M., Younkin L. H., and Younkin S. G. (1990) Expression of β amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer's disease using PCR.Neuron 4, 253–267.
Golde T. E., Estus S., Younkin L. H., Selkoe D. J., and Younkin S. G. (1992) Processing of the amyloid protein precursor to potentially amyloidogenic derivatives.Science 255, 728–730.
Goldman J. E., Yen S. H., Chiu F. C., and Peress N. S. (1983) Lewy bodies of Parkinson's disease contain neurofilament antigens.Science 221, 1082–1084.
Goldman J. E. and Yen S. H. (1986) Cytoskeletal protein abnormalities in neurodegenerative diseases.Ann. Neurol. 19, 209–223.
Goldstein M. E., Cooper H. S., Bruce J., Carden M. J., Lee V. M.-Y., and Schlaepfer W. W. (1987) Phosphorylation of neurofilament proteins and chromatolysis following transection of rat sciatic nerve.J. Neurosci. 7, 1586–1594.
Greenberg S. G. and Davies P. (1990) A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis.Proc. Natl. Acad. Sci. USA 87, 5827–5831.
Greenberg S. G., Davies P., Scheim J. D. and Binder L. I. (1992) Hydrofluoric acid-treated τPHF proteins display the same biochemical properties as normal tau.J. Biol. Chem. 267, 564–569.
Greengard P. (1987) Neuronal phosphoproteins. Mediators of signal transduction.Mol. Neurobiol. 1, 81–119.
Griffin J. W., Hoffman P. N., Clark A. W., Carroll P. T., and Price D. L. (1978) Slow axonal transport of neurofilament proteins: impairment by β,β′-iminodipropionitrile administration.Science 202, 633–635.
Griffin J. W. and Price D. L. (1980) Proximal axonopathies induced by toxic chemicals, inExperimental and Clinical Neurotoxicology (Spencer P. S. and Schaumburg H. H., eds.), Williams and Wilkins, Baltimore, MD, pp. 161–178.
Griffin J. W., Fahnestock K. E., Price D. L., and Hoffman P. N. (1983) Microtubule neurofilament segregation produced by β,β′-iminodipropionitrile: evidence for the association of fast axonal transport with microtubules.J. Neurosci. 3, 557–566.
Grundke-Iqbal I., Iqbal K., Tung M., Quinlan H., Wisniewski H., and Binder L. (1986) Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology.Proc. Natl. Acad. Sci. USA 83, 4913–4917.
Gundersen G. G., Halnoski M. H., and Bulinski J. C. (1984) Distinct populations of microtubules: tyrosinated and non-tyrosinated alpha tubulin are distributed differently in vivo.Cell 38, 779–789.
Gundersen G. G., Khawaja S., and Bulinski J. C. (1987) Post-polymerization detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules.J. Cell Biol. 105, 251–264.
Gustke N., Steiner B., Mandelkow E. M., Biernat J., Meyer H. E., Goedert M., and Mandelkow E. (1992) Tau protein-microtubule binding decreases upon Alzheimer-like phosphorylation at ser-pro motifs.FEBS Lett. 307, 199–205.
Haass C., Hung A. Y., and Selkoe D. J. (1991) Processing of β-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion.J. Neurosci. 11, 3783–3793.
Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B., and Selkoe D. J. (1992) Amyloid β-peptide is produced by cultured cells during normal metabolism.Nature 359, 322–325.
Halpain S. and Greengard P. (1990) Activation of NMDA receptors induces rapid dephosphorylation of the cytoskeletal protein MAP2.Neuron 5, 237–246.
Halpain S. and Greengard P. (1992) Protein dephosphorylation as a mediator of NMDA receptor signal transduction, inExcitatory Amino Acids and Second Messenger Systems (Teichberg V. I. and Turski L., eds.)., Springer-Verlag, Berlin, pp. 121–142.
Hansen L., Slamon D., Galasko D., Masliah E., Katzman R., DeTeresa R., Thal L., Pay M. M., Hofstetter R., Klauber M., Rice V., Butters N., and Alford M. (1990) The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity.Neurology 40, 1–8.
Hart C. E., Nuckolls G. H., and Wood J. G. (1987) Subcellular compartmentalization of phosphorylated neurofilament polypeptides in neurons.Cell Motil. Cytoskel. 7, 393–403.
Heidemann S. R., Landers J. M., and Hamborg M. A. (1981) Polarity orientation of axonal microtubules.J. Cell Biol. 91, 661–665.
Hill W. D., Lee V. M.-Y., Hurtig, H. I., Murray J. M., and Trojanowski J. Q. (1991) Epitopes located in spatially separate domains of each neurofilament subunit are present in Parkinson's disease Lewy Bodies.J. Comp. Neurol. 309, 150–160.
Himmler A., Drechsel D., Kirschner M., and Martin D. W., Jr. (1989) Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains.Mol. Cell Biol. 9, 1381–1388.
Hirokawa N. (1982) Cross-linker system between neurofilaments, microtubules and membranous organelles in frog axons revealed by the quckfreeze, deep-etch method.J. Cell Biol. 94, 129–142.
Hirokawa N., Glicksman M. A., and Willard M. B. (1984) Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton.J. Cell Biol. 98, 1523–1536.
Hirokawa N., Bloom G. S., and Vallee R. B. (1985) Cytoskeletal architecture and immunocytochemical localization of microtubule-associated proteins in regions of axons associated with rapid axonal transport: the β,β′-iminodipropionitrile-intoxicated axon as a model system.J. Cell Biol. 101, 227–239.
Hirokawa N., Hisanaga S. I., and Shiomura Y. (1988a) MAP2 is a component of crossbridges between microtubules and neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etch immunoelectron microscopy and reconstitution studies.J. Neurosci.8, 2769–2779.
Hirokawa N., Shiomura Y., and Okabe S. (1988b) Tau proteins: the molecular structure and mode of binding on microtubules.J. Cell Biol. 107, 1449–1459.
Hisanaga S. I. and Hirokawa N. (1988) Structure of the peripheral domains of neurofilaments revealed by low angle rotary shadowing.J. Mol. Biol. 20, 297–305.
Hisanaga S. I. and Hirokawa N. (1989) The effects of dephosphorylation on the structure of the projections of neurofilament.J. Neurosci. 9, 959–966.
Hoffman P. N. and Lasek R. J. (1975) The slow component of axonal transport: identification of the major structural polypeptides of the axon and their generality among mammalian neurons.J. Cell Biol. 66, 351–366.
Holtzman D. M., Li Y., DeArmond S. J., McKinley M. P., Gage F. H., Epstein C. J., and Mobley W. C. (1992) Mouse model of neurodegeneration: atrophy of basal forebrain cholinergic neurons in trisomy 16 transplants.Proc. Natl. Acad. Sci. USA 89, 1383–1387.
Hoshi M., Nishida E., Inagaki M., Gotoh Y., and Sakai H. (1990) Activation of a serine/threonine kinase that phosphorylates microtubule-associated protein 1B in vitro by growth factors and phorbol esters in quiescent rat fibroblastic cells.Eur. J. Biochem. 193, 513–519.
Hsiao K. K., Scott M., Foster D., Groth D. F., DeArmond J., and Prusiner S. B. (1990) Spontaneous neurodegeneration in transgenic mice with mutant prion protein.Science 250, 1587–1590.
Huber G. and Matus A. (1984) Immunocytochemical localization of microtubule-associated protein 1 in rat cerebellum using monoclonal antibodies.J. Cell Biol. 98, 777–781.
Hugon J. and Vallat J. M. (1990) Abnormal distribution of phosphorylated neurofilaments in neuronal degeneration induced by kainic acid.Neurosci. Lett. 119, 45–48.
Ihara Y. (1988) Massive somatodendritic sprouting of cortical neurons in Alzheimer's disease.Brain Res. 459, 138–144.
Johnson G. V. W., Watson A. L., Lartius R, Uemura E., and Jope R. S. (1992) Dietary aluminum selectively decreases MAP-2 in brains of developing and adult rats.Neurotoxicology 13, 463–474.
Joly J. C., Flynn G., and Purich D. L. (1989) The microtubule-binding fragment of microtubule-associated protein-2: location of the protease-accessible site and identification of an assembly-promoting peptide.J. Cell Biol. 109, 2289–2294.
Jucker M., Walker L. C., Martin L. J., Kitt C. A., Kleinman H. K., Ingram D. K., and Price D. L. (1992) Age-associated inclusions in normal and transgenic mouse brain.Science 255, 1443–1445.
Julien J.-P. and Mushynski W. E. (1982) Multiple phosphorylation sites in mammalian neurofilament polypeptides.J. Biol. Chem. 257, 10,467–10,470.
Julien J.-P. and Mushynski W. E. (1983) The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments.J. Biol. Chem. 258, 4019–4025.
Kammesheidt A., Boyce F. M., Spanoyannis A. F., Cummings B. J., Ortegon M., Cotman C., Vaught J. L., and Neve R. L. (1992) Deposition of β/A4 immunoreactivity and neuronal pathology in transgenic mice expressing the carboxyl-terminal fragment of the Alzheimer amyloid precursor in the brain.Proc. Natl. Acad. Sci. USA 89, 10,857–10,861.
Kanai Y., Takemura R., Oshima T., Mori H., Ihara Y., Yanagisawa M., Masaki T., and Hirokawa N. (1989) Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA.J. Cell Biol. 109, 1173–1184.
Kanai Y., Chen J., and Hirokawa N. (1992) Microtubule budling by tau proteins in vivo: analysis of functional domains.EMBO J. 11, 3953–3961.
Kang J., Lemaire H.-G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K.-H., Multhaup G., Beyreuther K., and Müller-Hill B. (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell surface receptor.Nature 325, 733–736.
Kaplan M. P., Chin S. S., Fliegner K. H., and Liem R. K. H. (1990) α-Internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain.J. Neurosci. 10, 2735–2748.
Katsetos C. D., Savory J., Herman M. M., Carpenter R. M., Frankfurter A., Hewitt C. D., and Wills M. R. (1990) Neuronal cytoskeletal lesions induced in the CNS by intraventricular and intravenous aluminum maltol in rabbits.Neuropathol. Appl. Neurobiol. 16, 511–528.
Kindler S., Schulz B., Goedert M., and Garner C. C. (1990) Molecular structure of microtubule-associated protein 2B and 2C from rat brain.J. Biol. Chem. 265, 19,679–19,684.
Klosen P., Anderton B. H., Brion J.-P., and van den Bosch de Aguilar P. (1990) Perikaryal neurofilament phosphorylation in axotomized and 6-OH-dopamine-lesioned CNS neurons.Brain Res. 526, 259–269.
Knops J., Kosik K. S., Lee G., Pardee J. D., Cohen-Gould L., and McConlogue L. (1991) Overexpression of tau in a non-neuronal cell induces long cellular processes.J. Cell Biol. 114, 725–733.
Koliatsos V. E., Applegate M. D., Kitt C. A., Walker L. C., DeLong M. R., and Price D. L. (1989) Aberrant phosphorylation of neurofilaments accompanies transmitter-related changes in septal neurons following transection of the fimbria-fornix.Brain Res. 482, 205–218.
Kondo J., Honea T., Mori H., Hamada Y., Miura R., Ogawara M., and Ihara, Y. (1988) The carboxyl third of tau is tightly bound to paired helical filaments.Neuron 1, 827–834.
Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fisher P., Master C. L., and Price D. L. (1990) Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport.Proc. Natl. Acad. Sci. USA 87, 1561–1565.
Kosik K. S., Joachim C. L., and Selkoe D. J. (1986) Mictotubule-associated protein τ (tau) is a major antigenic component of paired helical filaments in Alzheimer disease.Proc. Natl. Acad. Sci. USA 83, 4044–4048.
Kosik K. S., Orecchio L. D., Bakalis S., Duffy L., and Neve R. L. (1988) Partial sequence of MAP2 in the region of a shared epitope with Alzheimer neurofibrillary tangles.J. Neurochem. 51, 587–598.
Kosik K. S., Crandall J. E., Mufson E. J., and Neve R. L. (1989a) Tau in situ hybridization in normal and Alzheimer brain: localization in the somatodendritic compartment.Ann. Neurol. 26, 352–361.
Kosik K. S., Orecchio L. D., Bakalis S., and Neve R. L. (1989b) Developmentally regulated expression of specific tau sequences.Neuron 2, 1389–1397.
Kosik K. S. and Caceres A. (1991) Tau protein and the establishment of an axonal morphology.J. Cell Sci. Suppl. 15, 69–74.
Kosik K. S. (1992) Tau protein and neurodegeneration.Mol. Neurobiol. 4, 171–179.
Kosik K. S. (1993) The molecular and cellular biology of tau.Brain Pathol. 3, 39–43.
Kowall N. W. and Kosik K. S. (1987) Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease.Ann. Neurol. 22, 639–643.
Kreis T. E. (1987) Microtubules containing detyrosinated tubulin are less dynamic.EMBO J. 6, 2597–2606.
Ksiezak-Reding H. and Yen S.-H. (1987) Two monoclonal antibodies recognize Alzheimer's neurofibrillary tangles, neurofilament and microtubule associated proteins.J. Neurochem. 48, 455–462. with tau but show distinct biochemical properties.J. Neurosci. Res. 25, 420–430.
Ksiezak-Reding H., Chien C.-H., Lee V. M.-Y., and Yen S.-H. (1990b) Mapping of the Alz 50 epitope in microtubule-associated proteins tau.J. Neurosci. Res. 25, 412–419.
Ksiezak-Reding H. and Yen S. H. (1991) Structural stability of paired helical filaments requires microtubule-binding domains of tau: a model for self-association.Neuron 6, 717–728.
Kuzuhara S., Mori H., Izumiyama N., Yoshimura M., and Ihara Y. (1988) Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study.Neuropathologica 75, 345–353.
Langui D., Propst A., Anderton B., Brion J.-P., and Ulrich J. (1990) Aluminium-induced tangles in cultured rat neurons: enhanced effect of aluminium by addition of maltol.Acta Neuropathol. 80, 649–655.
LeClerc N., Kosik K. S., Cowan N., Pienkowski T. P., and Baas P. W. (1993) Process formation in Sf9 cells induced by the expression of a microtubule-associated protein 2C-like construct.Proc. Natl. Acad. Sci. USA 90, 6223–6227.
Lee V. M.-Y., Otvos L., Jr., Schmidt M. L., and Trojanowski J. Q. (1988a) Alzheimer's neurofibrillary tangles share immunological homologies with multiphosphorylation domains in the two large neurofilament proteins.Proc. Natl. Acad. Sci. USA 85, 7384–7388.
Lee G., Cowan N., and Kirschner M. (1988b) The primary structure and heterogeneity of tau protein from mouse brain.Science 239, 285–288.
Lee G., Neve R. L., and Kosik K. S. (1989) The microtubule binding domain of tau protein.Neuron 2, 1615–1624.
Lee V. M.-Y., Carden M. J., Schlaepfer W. W., and Trojanowski J. Q. (1987) Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats.J. Neurosci. 7, 3474–3488.
Lee V. M.-Y., Balin B. J., Otvos L., Jr., and Trojanowski J. (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal tau.Science 251, 675–678.
Leigh P. N., Propst A., Dale G., Power D., Brion J. P., Dodson A., and Anderton B. H. (1989) New aspects of the pathology of neurodegenerative disorders as revealed by ubiquitin antibodies.Neuropathology 79, 61–72.
Leonard G. B., Gorham D. G., Cole P., Greene L. A., and Ziff E. B. (1988) A nerve growth factorregulated messenger RNA encodes a new intermediate filament protein.J. Cell Biol. 106, 181–193.
Letterier J. F., Liem R., and Shelanski M. (1982) Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intra-organellar bridging.J. Cell Biol. 95, 982–986.
Lewis S. A., Sherline P., and Cowan, N. J. (1986a) A cloned cDNA encoding MAP 1 detects a single copy gene in mouse and brain-abundant RNA whose level decreases during development.J. Cell Biol. 102, 2106–2114.
Lewis S. A., Villasante A., Sherline P., and Cowan N. J. (1986b) Brain-specific expression of MAP 2 detected using a cloned cDNA probe.J. Cell Biol. 102, 2098–2105.
Lewis S. A., Wang D., and Cowan N. J. (1988) Microtubule-associated protein MAP2 shares a microtubule-binding motif with tau protein.Science 242, 936–939.
Lewis S. A., Ivanov I. E., Lee G., and Cowan N. J. (1989) Organization of microtubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP 2 and tau.Nature 342, 498–505.
Lewis S. A. and Cowan N. J. (1990) Microtubule bundling.Nature 345, 674.
Lewy F. H. (1912) Paralysis agitans. I. Pathologische Anatomie, inHandbuch der Neurologie (Lewandowsky M., ed.), Springer, Berlin, pp. 920–933.
Liem R. K. H., Chin S. S. M., Moraru E., and Wang E. (1985) Monoclonal antibodies to epitopes on different regions of the 200,000 dalton neurofilament protein.Exp. Cell Res. 156, 419–428.
Lindwall G. and Cole R. D. (1984) Phosphorylation affects the ability of tau protein to promote microtubule assembly.J. Biol. Chem. 259, 5301–5305.
Liu W.-K., Ksiezak-Reding H., and Yen S.-H. (1991) Abnormal tau proteins from Alzheimer's disease brains: purification and amino acid analysis.J. Biol. Chem. 266, 21,723–21,727.
Liu W.-K., Moore W. T., Williams R. T., Hall F. L., and Yen S.-H. (1993) Application of synthetic phospho- and unphospho-peptides to identify phosphorylation sites in a subregion of the tau molecule, which is modified in Alzheimer's disease.J. Neurosci. Res. 34, 371–376.
Love S. and Nicoll J. A. R. (1992) Comparison of modified Bielschowsky silver impregnation and anti-ubiquitin immunostaining of cortical and nigral Lewy bodies.Neuropathol. Appl. Neurobiol. 18, 585–592.
Lowe, J., Mayer J. R., and Landon M. (1993) Ubiquitin in neurodegenerative diseases.Brain Pathol. 3, 55–65.
Lu Q. and Wood J. G. (1993) Functional studies of Alzheimer's disease tau protein.J. Neurosci. 13, 508–515.
Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., and Beyreuther K. (1985) Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels.EMBO J. 4, 2757–2763.
Matus A., Bernhardt R., and Hugh-Jones T. (1981) High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain.Proc. Natl. Acad. Sci. USA 78, 3010–3014.
Matus A. (1988) Microtubule-associated proteins: their potential role in determining neuronal morphology.Ann. Rev. Neurosci. 11, 29–44.
Matus A. (1991) Microtubule-associated proteins and neuronal morphogenesis.J. Cell Sci. Suppl. 15, 61–67.
Mercken M., Vandermeeren M., Lübke U., Six J., Boons J., Van de Voorde A., Martin J.-J., and Gheuens J. (1992) Monoclonal antibodies with selective specificity for Alzheimer tau are directed against phosphatase-sensitive epitopes.Acta Neuropathol. 84, 265–272.
Migheli A., Butler M., Brown K., and Shelanski M. L. (1988) Light and electron microscope localization of the microtubule-associated protein tau in rat brain.J. Neurosci. 8, 1846–1851.
Miller P., Walter V., Therkauf W. E., Vallee R. B., and DeCamilli P. (1982) Frozen tissue sections as an experimental system to reveal specific binding sites for the regulatory subunit of type II cAMP-dependent protein kinase in neurons.Proc. Natl. Acad. Sci. USA 79, 5562–5566.
Miller C., Brion J.-P., Calvert R., Chin T. K., Eagles P. A. M., Downes M. J., Flament-Durant J., Haugh M., Kahn J., Propst A., Ulrich J., and Anderton B. H. (1986) Alzheimer paired helical filaments share epitopes with neurofilament sidearms.EMBO J. 5, 269–276.
Monaco S., Wongmongkolrit T., Shearson C. M., Patton A., Schaetzle B., Autilio-Gambetti L., Gambetti P., and Sayre L. M. (1990) Giant axonopathy characterized by intermediate location of axonal enlargements and acceleration of neurofilament transport.Brain Res. 519, 73–81.
Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Titani K., and Ihara Y. (1993) Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments.Neuron 10, 1151–1160.
Muma N. A., Troncoso J. C., Hoffman P. N., Koo E. H., and Price D. L. (1988) Aluminum neurotoxicity: altered expression of cytoskeletal genes.Mol. Brain Res. 3, 115–122.
Neve R. L., Harris P., Kosik K. S., Kurnit D. M., and Donlon T. A. (1986) Identification of cDNA clones for the human microtubule-associated protein, tau, and chromosomal localization of the genes for tau and microtubule-associated protein 2.Mol. Brain Res. 1, 271–280.
Neve R. L., Kammesheidt A., and Hohmann C. F. (1992) Brain transplants of cells expressing the carboxyl-terminal fragment of the Alzheimer amyloid protein precursor cause specific neuropathology in vivo.Proc. Natl. Acad. Sci. USA 89, 3448–3452.
Nixon R. A., Clarke J. F., Logvinenko K. B., Tan M. K. H., Hoult M., and Grynspan F. (1990) Aluminum inhibits calpain-mediated proteolysis and induces human neurofilament proteins to form protease-resistant high molecular weight complexes.J. Neurochem. 55, 1950–1959.
Nixon R. A. and Sihag R. K. (1991) Neurofilament phosphorylation: a new look at regulation and function.Trend. Neurosci. 14, 501–506.
Nixon R. A. (1993) The regulation of neurofilament protein dynamics by phosphorylation: clues to neurofibrillary pathobiology.Brain Pathol. 3, 29–38.
Noble M., Lewis S. A., and Cowan N. J. (1989) The microtubule binding domain of microtubule-associated protein MAP1B contains a repeated sequence motif unrelated to that of MAP2 and tau.J. Cell Biol. 109, 3367–3376.
Nukina N. and Ihara Y. (1986) One of the antigenic determinants of paired helical filaments is related to tau protein.J. Biochem. 99, 1541–1544.
Nunez J. (1988) Immature and mature variants of MAP and tau proteins and neuronal plasticity.Trends Neurosci. 11, 477–479.
Oblinger M. M., Argasinski A., Wong J., and Kosik K. S. (1991) Tau gene expression in DRG neurons during development and regeneration.J. Neurosci. 11, 2453–2459.
Ohtsubo K., Izumiyama N., Kuzuhara S., Mori H., and Shimada H. (1990) Curly fibers are tau-positive strands in the pre- and post-synaptic neurites, consisting of paired helical filaments: observations by the freeze-etch and replica method.Acta Neuropathol. 81, 111–115.
Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., and Sinha S. (1989) The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is a protease nexin-II.Nature 341, 144–147.
Papasozomenos S. Ch. and Binder L. I. (1987) Phosphorylation determines two distinct species of tau in the central nervous system.Cell Motil. Cytoskeleton 8, 210–226.
Peng I., Binder L. I., and Black M. M. (1986) Biochemical and immunological analysis of cytoskeletal domains of neurons.J. Cell Biol. 102, 252–262.
Perry R. H., Irving D., Blessed G., Fairbairn A., and Perry E. K. (1990) Senile dementia of Lewy body type: a clinically and neuropathologically distinct form of Lewy body dementia in the elderly.J. Neurol. Sci. 95, 119–139.
Perry G., Kawai M., Tabaton M., Onorato M., Mulvihill P., Richey P., Morandi A., Connolly J. A., and Gambetti P. (1991) Neuropil threads of Alzheimer's disease show a marked alteration of the normal cytoskeleton.J. Neurosci. 11, 1748–1755.
Piperno G. and Fuller M. T. (1985) Monoclonal antibodies specific for an acetylated form of α-tubulin recognize antigens in cilia and flagella from a variety of organisms.J. Cell Biol. 101, 2085–2094.
Ponte P., Gonzalez-De Whitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F., and Cordell B. (1988) A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors.Nature 331, 525–537.
Propst F., Rosenberg M. P., Cork L. C., Kovatch R. M., Rauch S., Westphal H., Khillan J., Schulz N. T., VandeWoude G. F., and Newmann P. E. (1990) Neuropathological changes in transgenic mice carrying copies of a transcriptionally activated Mos protooncogene.Proc. Natl. Acad. Sci. USA 87, 9703–9707.
Quinn P. N. and Steiger M. J. (1991) Parkinson's disease: clinical and therapeutic aspects.Curr. Opin. Neurol. Neurosurg. 4, 331–336.
Quon D., Wang Y., Catalano R., Scardina J. M., Murakami K., and Cordell B. (1991) Formation of β-amyloid protein deposits in brains of transgenic mice.Nature 352, 239–241.
Richards S.-J., Waters J. J., Beyreuther K., Masters C. L., Wischik C. M., Sparkman D. R., White C. L., Abraham C. R., and Dunnett S. B. (1991) Transplants of mouse trisomy 16 hippocampus provide a model of Alzheimer's disease neuropathology.EMBO J. 10, 297–303.
Riederer B. and Matus A. (1985) Differential expression of distinct microtubule-associated proteins during brain development.Proc. Natl. Acad. Sci. USA 82, 6006–6009.
Riederer B., Cohen R., and Matus A. (1986) MAP5: a novel brain microtubule-associated protein under strong developmental regulation.J. Neurocytol. 15, 763–775.
Riederer B., Guadano-Ferraz A., and Innocenti G. M. (1991) Differences in distribution of microtubule-associated proteins 5a and 5b during development of cerebral cortex and corpus callosum in cats: dependence on phosphorylation.Dev. Brain Res. 56, 235–243.
Rosenblum J. S., Bramblett G. T., and Lee V. M.-Y. (1990) The selective phosphorylation of sites near the microtubule binding domain in normal tau may be an initial event leading to the formation of Alzheimer disease (AD) paired helical filaments (PHF).J. Cell Biol. 111, 435a.
Rosenfeld J., Dorman M. E., Griffin J. W., Sternberger L. A., Sternberger N. H., Price D. L., and Gold B. G. (1987) Distribution of neurofilament antigens after axonal injury.J. Neuropathol. Exp. Neurol. 46, 269–282.
Safaei R. and Fischer I. (1989) Cloning of a cDNA encoding MAP1B in rat brain: regulation of mRNA levels during development.J. Neurochem. 15, 763–775.
Sato-Yoshitake R., Shiomura Y., Miyasaka H., and Hirokawa N. (1989) Microtubule-associated protein 1B: molecular structure, localization, and phosphorylation-dependent expression in developing neurons.Neuron 3, 229–238.
Sayre L. M., Autilio-Gambetti L., and Gambetti P. (1985) Pathogenesis of experimental giant neuro-filamentous axonopathies: a unified hypothesis based on chemical modification of neurofilaments.Brain Res. Rev. 10, 69–83.
Schubert W., Prior R., Weidemann A., Dircksen H., Multhaup G., Masters C. L., and Beyreuther K. (1991) Localization of Alzheimer βA4 amyloid precursor protein at cell and peripheral synaptic sites.Brain Res. 563, 184–194.
Selkoe D. J., Liem R. K. H., Yen S.-H., and Shelanski M. L. (1979) Biochemical and immunological characterization of neurofilaments in experimental neurofibrillary degeneration induced by aluminum.Brain Res. 163, 235–252.
Selkoe D. J., Abraham C. R., Podlisny M. B., and Duffy L. K. (1986) Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease.J. Neurochem. 46, 1820–1834.
Seubert P., Vigo-Pelfrey C., Esch F., Lee M. G., Dovey H., Davis D. L., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C., McCormack R., Wolfert R., Selkoe D. J., Lieberburg I., and Schenk D. B. (1992) Isolation and quantitation of soluble Alzheimer's β-peptide from biological fluids.Nature 359, 325–327.
Seubert P., Oltersdorf T., Lee M. G., Barbour R., Blomquist C., Davis D. L., Bryant K., Fritz L. C., Galasko D., Thal L. J., Lieberburg I., and Schenk D. B. (1993) Secretion of β-amyloid precursor protein cleaved at the amino terminus of the β-amyloid peptide.Nature 361, 260–263.
Shin R.-W., Bramblett G. T., Lee V. M.-Y., and Trojanowski J. Q. (1993) Alzheimer disease A68 proteins injected into rat brain induce codeposits of β-amyloid, ubiquitin, and α1-antichymotrypsin.Proc. Natl. Acad. Sci. USA 90, 6825–6828.
Shiomura Y. and Hirokawa N. (1987) The molecular structure of microtubule-associated protein 1A (MAP1A) in vivo, and in vitro. An immunoelectron microscopy and quick-freeze, deep-etch study.J. Neurosci. 7, 1461–1469.
Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffa L. M., Cai X.-D., McKay D. M., Tintner R., Frangione B., and Younkin S. G. (1992) Production of the Alzheimer amyloid β protein by normal proteolytic processing.Science 258, 126–129.
Simchowicz T. (1911) Sur la signification des plaques séniles et sur la formule sénile de l'écorce cérébrale.Revue Neurologique 1, 221–227.
Sisodia S. S., Koo E. H., Beyreuther K., and Unterbeck A. (1990) Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing.Science 248, 492–495.
Sisodia S. S., Koo E. H., Hoffman P. N., Perry G., and Price D. L. (1993) Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system.J. Neurosci. 13, 3136–3142.
Sternberger L. A. and Sternberger N. H. (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ.Proc. Natl. Acad. Sci. USA 80, 6162–6170.
Sternberger N. H., Sternberger L. A., and Ulrich J. (1985) Aberrant neurofilament phosphorylation in Alzheimer disease.Proc. Natl. Acad. Sci. USA 82, 4274–4276.
Szendrei G. I., Lee V. M.-Y., and Otvos L., Jr. (1993) Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location.J. Neurosci. Res. 34, 243–249.
Tabaton M., Cammarata S., Mancardi G., Manetto V., Autilio-Gambetti L., Perry G., and Gambetti P. (1991) Ultrastructural localization of β-amyloid, τ, and ubiquitin epitopes in extracellular neurofibrillary tangles.Proc. Natl. Acad. Sci. USA 88, 2098–2102.
Takeda M., Tatebayashi Y., Tanimukai S., Nakamura Y., Tanaka T., and Nishimura T. (1991) Immunohistochemical study of microtubule-associated protein 2 and ubiquitin in chronically aluminum-intoxicated rabbit brain.Acta Neuropathol. 82, 346–352.
Taleghany N. and Oblinger M. M. (1992) Regional distribution and biochemical characteristics of high molecular weight tau in the nervous system.J. Neurosci. Res. 33, 257–265.
Tanzi R. E., McClatchey A. J., Lamperti E. D., Villa-Komaroff L., Gusella J. F., and Neve R. L. (1988) Protease inhibitor domain encoded by an amyloid precursor mRNA associated with Alzheimer's disease.Nature 331, 528–530.
Theurkauf W. and Vallee R. B. (1983) Extensive cAMP-dependent and cAMP-independent phosphorylation of microtubule associated protein 2.J. Biol. Chem. 258, 7883–7886.
Trojanowski J. Q., Schuck T., Schmidt L. M., and Lee V. M.-Y. (1989) Distribution of tau proteins in the normal human central and peripheral nervous system.J. Histochem. Cytochem. 37, 209–215.
Troncoso J. C., Price D. L., Griffin J. W., and Parhad I. M. (1982) Neurofibrillary axonal pathology in aluminum intoxication.Ann. Neurol. 12, 278–283.
Troncoso J. C., Hoffman P. N., Griffin J. W., Hess-Kozlow K. M., and Price D. L. (1985) Aluminum intoxication: a disorder of neurofilament transport in motor neurons.Brain Res. 342, 172–175.
Troncoso J. C., Sternberger N. H., Sternberger L. A., Hoffman P. N., and Price D. L. (1986) Immunocytochemical studies of neurofilament antigens in the neurofibrillary pathology induced by aluminum.Brain Res. 364, 295–300.
Troncoso J. C., March J. L., Häner M., and Aebi U. (1990) Effect of aluminum and other multivalent cations on neurofilamentsin vitro: an electron microscopic study.J. Struct. Biol. 103, 2–12.
Tsao H., Aletta J. M., and Greene L. A. (1990) Nerve growth factor and fibroblast growth factor selectively activate a protein kinase thatphosphorylates high molecular weight microtubule-associated proteins.J. Biol. Chem. 265, 15,471–15,480.
Tsuyama S., Bramblett G. T., Huang K.-P., and Flavin M. (1986) A calcium/phospholipid-dependent kinase recognizes sites in microtubule associated protein 2 which are phosphorylated in living brain and are not accessible to other kinases.J. Biol. Chem. 261, 4110–4116.
Tsuyama S., Terayama Y., and Matsuyana S. (1987) Numerous phosphates of microtubule-associated protein 2 in living rat brain.J. Biol. Chem. 262, 10,886–10,892.
Tucker R. P., Binder L. I., Viereck C., Hemmings B. A., and Matus A. (1988) The sequential appearance of low- and high-molecular weight forms of MAP2 in the developing cerebellum.J. Neurosci. 8, 4503–4512.
Uéda K., Masliah E., Saitoh T., Bakalis S. L., Scoble H., and Kosik K. S. (1990) Alz-50 recognizes a phosphorylated epitope of tau protein.J. Neurosci. 10, 3295–3304.
Vermersch P., Frigard B., and Delacourte A. (1992) Mapping of neurofibrillary degeneration in Alzheimer's disease: evaluation of heterogeneity using the quantification of abnormal tau proteins.Acta Neuropathol. 85, 48–54.
Wakayama I., Nerurkar V. R., and Garruto R. M. (1993) Immunocytochemical and ultrastructural evidence of dendritic degeneration in motor neurons of aluminum-intoxicated rabbits.Acta Neuropathol. 85, 122–128.
Watson D. F., Griffin J. W., Fittro K. P., and Hoffman P. N. (1989) Phosphorylation-dependent immunoreactivity of neurofilaments increases during axonal maturation and β,β′-iminodipropionitrile intoxication.J. Neurochem. 53, 1818–1829.
Weingarten M. D., Lockwood A. H., Hwo S.-Y., and Kirschner M. W. (1975) A protein factor essential for microtubule assembly.Proc. Natl. Acad. Sci. USA 72, 1858–1862.
Willard M. and Simon C. (1981) Antibody decoration of neurofilaments.J. Cell Biol. 89, 198–205.
Wille H., Drewes G., Biernat J., Mandelkow E. M., and Mandelkow E. (1992a) Alzheimer-like paired helical filaments and anti-parallel dimers formed from microtubule-associated protein tau in vitro.J. Cell Biol. 118, 573–584.
Wille H., Mandelkow E. M., Dingus J., Vallee R. B., Binder L. I., and Mandelkow E. (1992b) Domain structure and antiparallel dimers of microtubule-associated protein 2 (MAP2).J. Struct. Biol. 108, 49–61.
Wirak D. O., Bayney R., Ramabhadran T. V., Fracasso R. P., Hart J. T., Hauer P. E., Hsiau P., Pekar S. K., Scangos G. A., Trapp B. D., and Unterbeck A. J. (1991) Deposits of amyloid β protein in the central nervous system of transgenic mice.Science 253, 323–325.
Wischik C. M., Novak M., Thogersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., and Klug A. (1988a) Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease.Proc. Natl. Acad. Sci. USA 85, 4506–4510.
Wischik C. M., Novak M., Edwards P. C., Klug A., Tichelaar W., and Crowther R. A. (1988b) Structural characterization of the core of the paired helical filament of Alzheimer disease.Proc. Natl. Acad. Sci. USA 85, 4884–4888.
Wolozin B. L., Pruchnicki A., Dickson D. W., and Davies P. (1986) A neuronal antigen in the brains of Alzheimer patients.Science 232, 648–650.
Wood J. G., Mirra S. S., Pollock N. J., and Binder L. I. (1986) Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau.Proc. Natl. Acad. Sci. USA 83, 4040–4043.
Yamaguchi H., Ishiguro K., Shoji M., Yamazaki T., Nakazato Y., Ihara Y., and Hirai S. (1990) Amyloid β/A4 protein precursor is bound to neurofibrillary tangles in Alzheimer-type dementia.Brain Res. 537, 318–322.
Yamaguchi H., Yamazaki T., Ishiguro K., Shoji M., Nakazato Y., and Hirai S. (1992) Ultrastructural localization of Alzheimer amyloid β/A4 protein precursor in the cytoplasm of neurons and senile plaque-associated astrocytes.Acta Neuropathol. 85, 15–22.
Yen S.-H., Dickson D. W., Crowe A., Butler M., and Shelanski M. L. (1987) Alzheimer neurofibrillary tangles contain unique epitopes in common with heat-stable microtubule associated proteins tau and MAP2.Am. J. Pathol. 126, 81–91.
Zang H., Sternberger N. H., Rubinstein L. J., Herman M. M., Binder L. I., and Sternberger L. A. (1989) Abnormal processing of multiple proteins in Alzheimer disease.Proc. Natl. Acad. Sci. USA 86, 8045–8049.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doering, L.C. Probing modifications of the neuronal cytoskeleton. Mol Neurobiol 7, 265–291 (1993). https://doi.org/10.1007/BF02769179
Issue Date:
DOI: https://doi.org/10.1007/BF02769179