Abstract
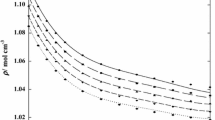
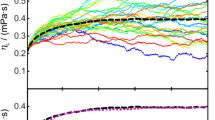
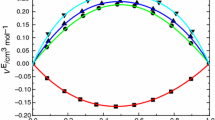
Densities and viscosities of binary mixtures (H2O or D2O) (1) + (DMSO or DMSO-D6)(2) have been measured over the entire mole fraction range; and the excess volumes, excess viscosities, and excess partial molar volumes Vf of the components have been obtained. All systems show negative excess volume Ve at all compositions, values for mixtures containing D2O being more negative than those with H2O byca. 0.03 cm3-mol-1 at x1, = 0.6, where a minimum is observed. The difference between DMSO and DMSO-D6 containing mixtures is negligible. The excess viscosity ηe is always positive and shows a maximum at x1 = 0.65; at this composition, the substitution of H2O with D2O causes an excess viscosity increment ofca. 0.35 mPa-s, while deuteration of DMSO brings about a smaller increase,ca. 0.1 mPa-s. The trend of V E2 with concentration shows the characteristic features of moderately hydrophobic solutes in water (negative values and a minimum in the water-rich region), features that are slightly but significantly more marked in D2O than in H2O. The V E2 values in the water-diluted region and at x1, =0 are more negative for D2O than for H2O.
Similar content being viewed by others
References
R. A. Home, Ed.,Water and Aqueous Solutions. Structure, Thermodynamics and Transport Processes, (Wiley, New York, 1972.)
J. M. G. Cowie and P. M. Toporowski,Can. J. Chem. 39, 2240 (1961).
R. G. Lebel and D. A. Goring,J. Chem. Eng. Data 7, 100 (1962).
O. Kiyohara, G. Perron, and J. E. Desnoyers,Can. J. Chem. 53, 3263 (1975).
L. Werblan and J. Lesinski,Pol. J. Chem. 52, 1211 (1978).
C. De Visser, W. J. M. Heuvelsland, L. A. Dunn, and G. Somsen,Trans. Faraday Soc. 74, 1159(1978).
O. Iulian, C. Meteescu, and M. Jliuta,Rev. Rom. Chem. 40, 235 (1995).
T. M. Aminabhavi and B. Gopalakrishna,J. Chem. Eng. Data 40, 856 (1995).
D. J. Pruett and L. K. Felker,J. Chem. Eng. Data 30, 452 (1985).
E. V. Ivanov and V. X. Abrossimov,J. Solution Chem. 25, 191 (1986).
T. C. Chan and W. A. Van Hook,J. Solution Chem. 5, 107 (1976).
Gy. Jakli and W. A. Van Hook,J. Chem. Thermodyn. 4, 857 (1972).
M. Holz, X. Mao, D. Seiferling, and A. Sacco,J. Chem. Phys. 104, 669 (1996).
H. Weingärtner, M. Holz, A. Sacco, and M. Trotta,J. Chem. Phys. 91, 2568 (1989).
A. Sacco and M. Holz, to be published.
Gy. Jakli and W. A. Van Hook,J. Chem. Eng. Data 41, 249 (1996).
F. Franks, Ed.,Water—A Comprehensive Treatise, Vol.2, (Plenum, New York, 1975).
M. Holz, R. Grander, A. Sacco, and A. Meleleo,J. Chem. Soc. Faraday Trans. 89, 1215 (1993).
C. Christensen, J. Gmehling, P. Rasmussen, and U. Weidlich,Heats of Mixing Data Collection, Binary Systems, Chemistry Data Series, Vol. III, Part1, (Dechema, Frankfurt am Main, Germany, 1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sacco, A., Matteoli, E. Isotopic substitution effects on the volumetric and viscosimetric properties of water-dimethylsulfoxide mixtures at 25°C. J Solution Chem 26, 527–535 (1997). https://doi.org/10.1007/BF02767604
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02767604