Abstract
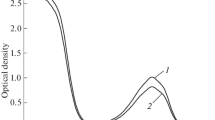
Adsorption-desorption isotherms of tetrachloromethane and water vapors were used to characterize the adsorption properties of porous tin silicophosphate prepared by coprecipitation from a solution of activated silicic acid and SnCl4 at Si : Sn molar ratios from 0.9 : 0.1 to 0.1 : 0.9. The adsorption isotherms obtained are characteristic of microor mesoporous adsorbents. The boundary between microand mesoporous microstructures lies at Si : Sn = 0.3 : 0.7. The isotherms of water vapor sorption are found to be irreversible over the entire range of relative pressures studied.
Similar content being viewed by others
References
Inorganic Ion Exchange Materials, Clearfield, A., Ed., Boca Raton: CRC., 1982.
Kuznetsova, T.F., Lemeshonok, G.S. and Eremenko, S.I., Pore Structure of Aluminum Zirconium Phosphate,Zh. Fiz. Khim., 1998, vol. 72, no. 7, pp. 1248–1251.
Kuznetsova, T.F., Barkatina, E.N., Lemeshonok, G.S., and Eremenko, S.I., Preparation of Mixed Layered Hydroxides of Di- and Tri-valent Metals via Sequential Cation Precipitation,Kolloidn. Zh., 1996, vol. 58, no. 2, pp. 215–219.
Kuznetsova, T.F., Burdovitsyna, L.I., Lemeshonok, G.S., and Eremenko, S.I., Effect of Oxoanions on the Sorption Properties of Nickel Chromium Hydroxides,Kolloidn. Zh., 1996, vol. 58, no. 5, pp. 623–626.
Kuznetsova, T.F. and Burdovitsyna, L.I., Sorption and Structural Properties of the Sequentially Precipitated Nickel-Chromium Hydroxides,Appl. Catal., 1997, vol. 152, no. 1, pp. 1–6.
Iler, R.,The Chemistry of Silica, New York: Wiley, 1979. Translated under the titleKhimiya kremnezema, Moscow: Mir, 1982.
Gurvich, L.G., On the Physicochemical Attracting Force,Zh. Russk. Fiz.-Khim. O-va. Imperator. Petrogr. Univ., Ch. Khim, 1915, vol. 47, no. 4, pp. 805–827.
McClellan, A.L. and Harnsberger, H.E., Cross-Sectional Areas of Molecules Adsorbed on Solid Surfaces,J. Colloid Interface Sci., 1967, vol. 23, pp. 577–599.
IUPAC: Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity,Pure Appl. Chem., 1985, vol. 57, no. 4, pp. 603–619.
Gregg, S.J. and Sing, K.S.W.,Adsorption, Surface Area, and Porosity, New York: Academic, 1982. Translated under the titleAdsorbtsiya, udel’naya poverkhnost’, poristost’, Moscow: Mir, 1984.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuznetsova, T.F., Eremenko, S.I. & Lemeshonok, G.S. Adsorption properties of tin silicophosphate. Inorg Mater 36, 932–934 (2000). https://doi.org/10.1007/BF02758707
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02758707