Abstract
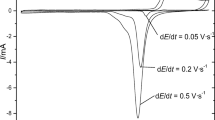
The dicarbollyliron (Cb2Fe-) redox reaction is studied at an amalgamated platinum electrode coated with a monolayer of behenic acid on top of which hemin is adsorbed. The redox reaction of Cb2Fe- involves two stages. First, an electrochemical reaction of adsorbed hemin proceeds, which involves the electron transfer through the dielectric monolayer; it is followed by the hemin’s chemical redox reaction with dissolved Cb2Fe-. It is shown that the adsorbed hemin transformation, no matter how small, is sufficient for the stimulation of the Cb2Fe- redox reaction.
Similar content being viewed by others

References
Khanova, L.A. and Krishtalik, L.I.,Elektrokhimiya, 1998, vol. 34, p. 5.
Khanova, L.A.,Elektrokhimiya, 1999, vol. 35, p. 618.
Khanova, L.A. and Tarasevich, M.R.,Elektrokhimiya, 1995, vol. 31, p. 212.
Ksenzhek, O.S. and Petrova, S.A.,Bioelectrochem. Bioenerg., 1978, vol. 5, p. 661.
Bagotzky, V.S. and Yablokova, I.E.,Th. Fiz, Khim., 1953, vol. 27, p. 1663.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khanova, L.A. Two-stage mechanism of the dicarbollyliron redox-reaction at electrode coated with a monolayer of behenic acid and hemin. Russ J Electrochem 36, 54–59 (2000). https://doi.org/10.1007/BF02757796
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02757796