Summary
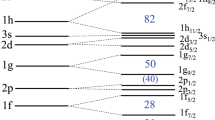
A minimum condition on a suitable function, built with the atom energy level values from Dirac’s formula, produces coincidences involving the numbers, given by Pauli’s principle, that describe the electron arrangement inside the atom shells.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bottini, S. Some coincidences concerning the electron occupancy numbers of atom subshells. Lett. Nuovo Cimento 42, 134–136 (1985). https://doi.org/10.1007/BF02748347
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02748347