Abstract
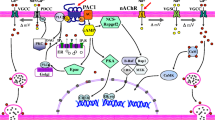
Pituitary adenylate cyclase activating polypeptide (PACAP) elevates levels of the mRNAs encoding the catecholamine synthesizing enzymes tyrosine hydroxylase (TH), dopamine β-hydroxylase (DBH), and phenylethanolamineN-methyltransferase (PNMT) in primary cultures of bovine adrenal chromaffin cells. PACAP potently (in nanomolar concentrations) increases the amount of mRNA for each of the three catecholamine biosynthetic enzymes. At 10 nM PACAP, TH and DBH mRNA levels increase approx 10-fold; 1 nM PACAP produces an approx 2.5-fold elevation of PNMT mRNA. In contrast to depolarizing or cholinergic stimuli, PACAP does not enhance expression of 5′ upstream regions of the PNMT gene transiently transfected into chromaffin cells. Nor does PACAP stimulate the rate of PNMT gene transcription, thereby indicating that the effects of this neuropeptide do not involve enhanced transcription of this gene. However, after 16 h in the presence of transcriptional inhibitors, more PNMT mRNA is present in cultures treated with PACAP relative to control cultures, whereas amounts of TH and DBH mRNAs are not changed. PACAP likely elevates PNMT mRNA levels posttranscriptionally, possibly by stabilizing this message against degradation. Thus, although PACAP is an effective regulator for expression of all three catecholamine enzyme genes, its mechanism of action on PNMT mRNA appears to be distinctive from its effects on TH and DBH gene transcription.
Similar content being viewed by others
References
Arimura A., Somogyvari-Vigh A., Miyata A., Mizuno K., Coy D. H., and Kitada C. (1991) Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes.Endocrinology 129, 2787–2789.
Arimura A. (1992) Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research.Reg. Peptides 37, 287–303.
Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., and Struhl K. (1991)Current Protocols in Molecular Biology, Wiley, New York.
Baetge E. E., Suh Y., and Joh T. H. (1986) Complete nucleotide and deducted amino acid sequence of bovine phenylethanolamine N-methyltransferase: partial amino acid homology with rat tyrosine hydroxylase.Proc. Natl. Acad. Sci. USA 83, 5454–5458.
Carroll J. M., Evinger M. J., Goodman H. M., and Joh T. H. (1991) Differential and coordinate regulation of TH and PNMT mRNAs in chromaffin cell cultures by second messenger system activation and steroid treatment.J. Mol. Neurosci. 3, 75–83.
Chowdhury P. S., Guo X., Wakade T. D., Przywara D. A., and Wakade A. R. (1994) Exocytosis from a single rat chromaffin cell by cholinergic and peptidergic neurotransmitters.Neuroscience 59, 1–5.
Deutsch P. J. and Sun Y. (1992) The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth.J. Biol. Chem. 267, 5108–5113.
Edlund T., Walker M. D., Barr P. J., and Rutter W. J. (1985) Cell specific expression of the rat insulin gene: evidence for the role of two distinct 5′ flanking elements.Science 230, 912–916.
Evinger M. J. and Joh T. H. (1989) Strain-specific differences in transcription of the gene for the epinephrine-synthesizing enzyme phenylethanolamine N-methyltransferase.Mol. Brain Res. 5, 141–147.
Evinger M. J., Joh T. H., and Reis D. J. (1990) Transcriptional regulation of phenylethanolamine N-methyltransferase gene expression, inCatecholamine Genes (Joh T. H., ed.), Liss, New York, pp. 137–146.
Evinger M. J., Hemmick L. M., Regunathan S., Reis D. J., and Ross M. E. (1992a) Nicotine and muscarine stimulate expression of PNMT promoter constructs transfected into primary chromaffin cells.Soc. Neurosci. Abst. 18, 254.3.
Evinger M. J., Towle A. C., Park D. H., Lee P., and Joh T. H. (1992b) Glucocorticoids stimulate transcription of the rat phenylethanolamine N-methyltransferase (PNMT) gene in vivo and in vitro.Cell. Mol. Neurobiol. 12, 193–215.
Evinger M. J., Ernsberger P., Regunathan S., Joh T. H., and Reis D. J. (1994) A single transmitter regulates gene expression through two separate mechanisms: Cholinergic regulation of PNMT mRNA via nicotinic and muscarinic pathways.J. Neurosci. 14, 2106–2116.
Evinger M. J., Ernsberger P., Regunathan S., and Reis D. J. (1995) Regulation of phenylethanolamine N-methyltransferase gene expression by imidazoline receptors in adrenal chromaffin cells.J. Neurochem. 65, 988–997.
Fossom L. H., Carlson C. D., and Tank A. W. (1991) Stimulation of tyrosine hydroxylase gene transcription rate by nicotine in rat adrenal medulla.Mol. Pharm. 40, 193–202.
Guo X. and Wakade A. R. (1994) Differential secretion of catecholamines in response to peptidergic and cholinergic transmitters in rat adrenals.J. Physiol. (Lond.) 475, 539–545.
Hashimoto H., Ishihara T., Shigemoto R., Mori K., and Nagata S. (1993) Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide.Neuron 11, 333–342.
Hemmick L. M. and Evinger M. J. (1993) Muscarinic activation of PNMT gene expression maps to upstream elements.Soc. Neurosci. Abstr. 19, 520.1.
Hentze M. (1991) Determinants and regulation of cytoplasmic mRNA stability in eukaryotic cells.Biochim. Biophys. Acta. 1090, 281–292.
Hwang O. and Joh T. H. (1993) Effects of cAMP, glucocorticoids, and calcium on dopamine beta-hydroxylase gene expression in bovine chromaffin cells.J. Mol. Neurosci. 4, 173–183.
Kilbourne E. J., Nankova B. B., Lewis E. J., McMahon A., Osaka H., Sabban D. B., and Sabban E. L. (1992) Regulated expression of the tyrosine hydroxylase gene by membrane depolarization.J. Biol. Chem. 267, 7563–7569.
Kim K. S., Ishiguro H., Tinti C., Wagner J., and Joh T. H. (1994) The cAMP-dependent protein kinase regulates transcription of the dopamine beta-hydroxylase gene.J. Neurosci. 14, 7200–7207.
Kimura C., Ohkubo S., Ogi K., Hosoya M., Itoh Y., Onda H., Miyata A., Jiang L., Dahl R. R., Stibbs H. H., Arimura A., and Fujino M. (1990) A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the bovine and human cDNAs.Biochem. Biophys. Res. Commun. 166, 81–89.
Kumakura K., Guidotti A., and Costa E. (1979) Primary cultures of chromaffin cells: molecular mechanisms for the induction of tyrosine hydroxylase mediated by 8-Br-cyclic AMP.Mol. Pharmacol. 16, 865–876.
Lewis E. J., Harrington C. A., and Chikaraishi D. M. (1987) Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoids and cyclic AMP.Proc. Natl. Acad. Sci. USA 84, 3550–3554.
McMahon A. and Sabban E. L. (1992) Regulation of expression of dopamine beta-hydroxylase in PC12 cells by glucocorticoids and cyclic AMP analogs.J. Neurochem. 59, 2040–2047.
Marley P. D. and McLeod J. (1995) Localization of PACAP-27 immunoreactivity in nerves of bovine and rat adrenal glands.Eighth International Symposium on Chromaffin Cell Biol., Abstr. P-74.
Masuo Y., Suzuki N., Matsumoto H., Tokito F., Matsumoto Y., Tsuda M., and Fujino M. (1993) Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay.Brain. Res. 602, 57–63.
May V. and Braas K. M. (1995) Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptideY and catecholamine expression.J. Neurochem. 65, 978–987.
Miyata A., Arimura A., Dahl R. R., Minamino N., Uehara A., Jiang L., Culler M. D., and Coy D. H. (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells.Biochem. Biophys. Res. Commun. 164, 567–574.
Miyata A., Jiang L., Dahl R. R., Kitada C., Kubo K., Fujino M., Minamino N., and Arimura A. (1990) Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues.Biochem. Biophys. Res. Commun. 170, 643–648.
Nordeen S. K. (1988) Luciferase reporter gene vectors for analysis of promoters and enhancers.Biotechniques 6, 454–457.
Ogi K., Kimura C., Onda H., Arimura A., and Fujino M. (1990) Molecular cloning and characterization of cDNA for the precursor of rat pituitary adenylate cyclase activating polypeptide (PACAP).Biochem. Biophys. Res. Commun. 173, 1271–1279.
Pisegna J. R. and Wank S. A. (1996) Cloning and characterization of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C.J. Biol. Chem. 271, 17,267–17,274.
Przywara D. A., Guo X., Anelilli M. L., Wakade T. D., and Wakade A. R. (1996) A non-cholinergic transmitter, pituitary adenylate cyclase-activating polypeptide, utilizes a novel mechanism to evoke catecholamine secretion in rat adrenal chromaffin cells.J. Biol. Chem. 271, 10,545–10,550.
Przywara D. A., Guo X., and Wakade A. R. (1995) A non-cholinergic transmitter, PACAP, uses a novel mechanism to evoke catecholamine secretion.Eighth International Symposium on Chromaffin Cell Biol. Abstr. P-86.
Rius R. A., Guidotti A., and Costa E. (1994) Pituitary adenylate cyclase activating polypeptide (PACAP) potently enhances tyrosine hydroxylase (TH) expression in adrenal chromaffin cells.Life Sci. 54, 1735–1743.
Ross M. E., Evinger M. J., Hyman S. E., Carroll J. M., Mucke L., Comb M., Reis D. J., Joh T. H., and Goodman H. M. (1990) Identification of a functional glucocorticoid response element in the phenylethanolamine N-methyltransferase promoter using fusion genes introduced into chromaffin cells in primary culture.J. Neurosci. 10, 520–530.
Sabban E. L. and Nankova B. (1997) Multiple pathways in regulation of dopamine β-hydroxylase, inAdvances in Pharmacology vol.42 (Goldstein D., ed.), Academic, New York, in press.
Sassone-Corsi P. (1995) Transcription factors responsive to cAMP.Ann. Rev. Cell. Dev. Biol. 11, 355–377.
Shiotani Y., Kimura S., Ohshige Y., Yanaihara C., and Yanaihara N. (1995) Immunohistochemical localization of pituitary adenylate cyclase-activating polypeptide (PACAP) in the adrenal medulla of the rat.Peptides 16, 1045–1050.
Shivers B. D., Gorcs T. J., Gottschall P. E., and Arimura A. (1991) Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions.Endocrinology 128, 3055–3065.
Spengler D., Waeber C., Pantaloin C., Holsboer F., Bockaert J., Seeburg P. H., and Journot L. (1993) Differential signal transduction by five splice variants of the PACAP receptor.Nature 365, 170–175.
Stachowiak M. K., Hong J. S., and Viveros O. H. (1990) Coordinate and differential regulation of phenylethanolamine N-methyltransferase, tyrosine hydroxylase, and proenkephalin mRNAs by neural and hormonal mechanisms in cultured bovine adrenal medullary cells.Brain Res. 510, 277–288.
Stachowiak M. K., Goc A., Hong J.-S., Poisner A., Jiang H.-K., and Stachowiak E. K. (1994) Regulation of tyrosine hydroxylase gene expression in depolarized non-transformed bovine adrenal medullary cells: second messenger systems and promoter mechanisms.Mol. Brain Res. 22, 309–319.
Strong R., Wakade A. R., Arimura A., and Haycock J. W. (1992) Short- and long-term effects of PACAP in PC 12 cells: Phosphorylation and induction of tyrosine hydroxylase.Reg. Peptides 37, 332 (Abstract).
Tank A. W., Curella P., and Ham L. (1986) Induction of mRNA for tyrosine hydroxylase by cyclic AMP and glucocorticoids in a rat pheochromocytoma cell line.Mol. Pharmacol. 30, 497–503.
Tischler A. S., Riseberg J. C., and Gray R. (1995) Mitogenic and antimitogenic effects of pituitary adenylate cyclase-activating polypeptide (PACAP) in adult rat chromaffin cell cultures.Neurosci. Lett. 189, 135–138.
Villalba M., Bockaert J., and Journot L. (1997) Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects/cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway.J. Neurosci. 17, 83–90.
Wakade A. R. (1988) Noncholinergic transmitter(s) maintain(s) secretion of catecholamines from rat adrenal medulla for several hours of continuous stimulation of splanchnic neurons.J. Neurochem. 50, 1302–1308.
Wakade A. R., Guo X., Strong R., Arimura A., and Haycock J. W. (1992) Pituitary adenylate cyclase-activating polypeptide (PACAP) as a neurotransmitter in rat adrenal medulla.Reg. Peptides 37, 331 (Abstract).
Watanabe T., Ohtaki T., Kitada C., Tsuda M., and Fujino M. (1990) Adrenal pheochromocytoma PC12H cells respond to pituitary adenylate cyclase activating polypeptide.Biochem. Biophys. Res. Commun. 173, 252–258.
Watanabe T., Masuo Y., Matsumoto H., Suzuki N., Ohtaki T., Masuda Y., Kitada C., Tsuda M., and Fujino M. (1992) Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline.Biochem. Biophys. Res. Commun. 182, 403–411.
Wessels-Reiker M., Basiboina R., Howlett A. C., and Strong R. (1993) Vasoactive intestinal polypeptide-related peptides modulate tyrosine hydroxylase gene expression in PC12 cells through multiple adenylate cyclase-coupled receptors.J. Neurochem. 60, 1018–1029.
Zigmond R. E. (1988) A comparison of the long-term and short-term regulations of tyrosine hydroxylase activity.J. Physiol. 83, 267–271.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tönshoff, C., Hemmick, L. & Evinger, M.J. Pituitary adenylate cyclase activating polypeptide (PACAP) regulates expression of catecholamine biosynthetic enzyme genes in bovine adrenal chromaffin cells. J Mol Neurosci 9, 127–140 (1997). https://doi.org/10.1007/BF02736856
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02736856