Abstract
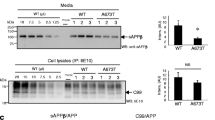
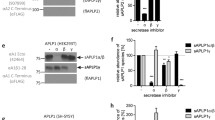
To study amyloid precursor protein (APP) processing we expressed different APP isoforms with and without the Swedish mutation and the membrane inserted C-terminal 100 residues of APP (SPA4CT) in the human neuroblastoma cell line SY5Y. We show that expression of the Swedish mutation results in a significant production of the amyloidogenic intermediate A4CT, which is further processed by γ-secretase leading to an overproduction of βA4. Treatment with methylamine and ammonium chloride, inhibitors interfering with intracellular transport mechanisms, inhibits β-secretase activity without influencing the physiological APP cleavage by α-secretase activity. By expressing SPA4CT, we demonstrate that secretion, but not generation, of βA4 from SPA4CT is inhibited by methylamine resulting in intracellular βA4. This provides experimental evidence for the intracellular localization of γ-secretase activity and βA4 generation.
Similar content being viewed by others
References
Busciglio J., Gabuzda D. H., Matsudaira P., and Yankner B. A. (1993) Generation of β-amyloid in the secretory pathway in neuronal and non neuronal cells.Proc. Natl. Acad. Sci. USA 9, 292–296.
Cai X., Golde T., and Younkin S. (1993) Release of excess amyloid β-protein from a mutant amyloid β-protein precursor.Science 259, 514–516.
Caporaso G. L., Gandy S. E., Buxbaum J. D., and Greengard P. (1992) Chloroquine inhibits intracellular degradation but not secretion of Alzheimer β/A4 amyloid precursor protein.Proc. Natl. Acad. Sci. USA 89, 2252–2256.
Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., and Selkoe D. J. (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production.Nature 360, 672–674.
De Strooper B., Umans L., Van Leuven F., and Van Den Berghe H. (1993) Study of the synthesis and secretion of normal and artificial mutants of murine amyloid precursor protein (APP): cleavage of APP occurs in a late compartment of the default secretion pathway.J. Cell Biol. 121, 234–295.
Dyrks T., Dyrks E., Master C. L., and Beyreuther K., (1992) Membrane inserted APP fragments containing the βA4 sequence of Alzheimer’s disease do not aggregate.FEBS Lett. 39, 2–24.
Dyrks T., Dyrks E., Mönning U., Turner J., and Beyreuther K. (1994) Amyloid precursor protein secretion and βA4 amyloid generation are not mutually exclusive.FEBS Lett. 349, 212–214.
Dyrks T., Dyrks E., Mönning U., Urmoneit B., Turner J., and Beyreuther K. (1993) Generation of βA4 from the amyloid protein precursor and fragments thereof.FEBS Lett. 335, 89–93.
Esch F. S., Keim P. L., Beattle E. C., Blacher R. W., Culwell A. R., Ottersdorf T., Mc Clure D., and Ward P. J. (1990) Cleavage of amyloid β peptide during constitutive processing of its precursor.Science 248, 1122–1124.
Haass C., Hung A. Y., Schlossmacher M. G., Teplow D. B., and Selkoe D. J. (1993) β-Amyloid peptide and 3-kDa fragment are derived by distinct cellular mechanisms.J. Biol. Chem. 268, 5321–5324.
Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B., and Selkoe D. J. (1992) Amyloid β-peptide is produced by cultured cells during normal metabolism.Nature 359, 322–325.
Kang J., Lemaire H.-G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K.-H., Multhaup G., Beyreuther K., and Müller-Hill B. (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor.Nature 325, 733–736.
Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., and Ito H. (1988) Novel precursor of Alzheimer’s disease amyloid protein shows protease inhibitory activity.Nature 331, 530–532.
Knops J., Lieberburg I., and Sinha S. (1992) Evidence for a nonsecretory, acidic degradation pathway for amyloid precursor protein in 293 cells.J. Biol. Chem. 23, 1622–1624.
König G., Mönning U., Czech C., Prior R., Banati R., Schreiter-Gasser U., Bauer J., Masters C. L., and Beyreuther K. (1992) Identification and differential expression of a novel alternative splice isoform of the βA4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells.J. Biol. Chem. 267, 184–189.
Lo A. C., Haas C., Wagner S. L., Teplow D., and Sisodia S. S. (1994) Metabolism of the “Swedish” amyloid precursor protein variant in Madia-Darby canine kidney cells.J. Biol. Chem. 269, 30,966–30,973.
Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenber B., Davis K., Wallace W., Lieberburg I., Fuller F., and Cordell B. (1988) A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors.Nature 311, 525–527.
Sambrook J., Fritsch E. F., and Maniatis T. (1989)Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X., McKay D. M., Tinter R., Frangione B., and Younkin S. G. (1992) Production of the Alzheimer amyloid β protein by normal proteolytic processing.Science 258, 126–129.
Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., and Price D. L. (1990) Evidence that β-amyloid protein in Alzheimer’s disease is not derived by normal processing.Science 248, 492–495.
Tanzi R. E., McClatchey A. I., Lamerti E. D., Villa-Komaroff L., Gusella J. F., and Neve R. L. (1988) Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer’s disease.Nature 311, 528–532.
Weidemann A., König G., Bunke D., Fischer P., Salbaum J.-M., Master C. L., and Beyreuther K. (1989) Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein.Cell 57, 115–126.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Urmoneit, B., Reinsch, C., Turner, J. et al. Inhibition of βA4 production by specific modulation of β-secretase activity. J Mol Neurosci 6, 23–32 (1995). https://doi.org/10.1007/BF02736756
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02736756