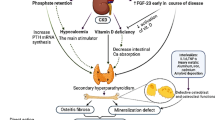
Abstract
Renal osteodystrophy is a common complication of chronic Kidney disease, and abnormalities of bone metabolism are a reflection of broad-based disturbances in the control mechanisms for mineral metabolism. Secondary hyperparathyroidism is a major contributor to the high turnover form of renal osteodystrophy. Detailed investigations over the past several decades have uncovered many of the mechanisms involved in the initiation and maintenance of secondary hyperparathyroidism and it is these mechanisms that provide the basis for therapeutic intervention to control this complication of chronic kidney disease. An additional form of renal osteodystropy is characterized by abnormally low bone turnover, which also is multi-factorial in origin. Again, an understanding of the mechanisms involved in abnormal bone and mineral homeostasis provide the basis for therapy. It is only with a through understanding of the mechanisms involved in the initiation and maintenance of these complications that rational approaches to treatment may be instituted.
Similar content being viewed by others
References
Monier-Faugere MC, Malluche HH. 1996 Trends in renal osteodystrophy: a survey from 1983 to 1995 in a total of 2248 patients. Nephrol Dial Transpl 11:111–120.
Malluche H, Monier-Faugere M. 1994 The role of bone biopsy in the management of patients with renal osteodystrophy. J Am Soc Nephrol 4:1631–1642.
Sherrard DJ, Hercz G, Pei Y, Maloney NA et al. 1993 The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int 43:436–442.
Reiss E, Canterbury JM, Kanter A. 1969 Circulating parathyroid hormone concentration in chronic renal insufficiency. Arch Intern Med 124:417–422.
Malluche H, Ritz E, Lange H. 1976 Bone histology in incipient and advanced renal failure. Kidney Int 9:355–362.
Rutherford WE, Bordier P, Marie P et al. 1977 Phosphate control and 25-hydroxycholecalciferol administration in preventing experimental renal osteodystrophy in the dog. J Clin Invest 60:332–341.
Slatopolsky E, Caglar S, Pennell JP et al. 1971 On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest 50:492–499.
Bricker NS. 1972 On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N Engl J Med 286:1093–1099.
Lopez-Hilker S, Galceran T, Chan YL et al. 1986 Hypocalcemia may not be essential for the development of secondary hyperparathyroidism in chronic renal failure. J Clin Invest 78:1097–1102.
Almaden Y, Canalejo A, Hernandez A et al. 1996 Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res 11:970–976.
Slatopolsky E, Finch J, Denda M et al. 1996 Phosphorus restriction prevents parathyroid gland growth. High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest 97:2534–2540.
Moallem E, Kilav R, Silver J, Naveh-Many T. 1998 RNA-protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J Biol Chem 273:5253–5259.
Yalcindag C, Silver J, Naveh-Many T. 1999 Mechanism of increased parathyroid hormone mRNA in experimental uremia: roles of protein RNA binding and RNA degradation. J Am Soc Nephrol 10:2562–2568.
Kilav R, Silver J, Naveh-Many T. 2001 A conserved cis-acting element in the parathyroid hormone 3′-untranslated region is sufficient for regulation of RNA stability by calcium and phosphate. J Biol Chem 276:8727–8733.
Almaden Y, Canalejo A, Ballesteros E, Anon G, Rodriguez M. 2000 Effect of high extracellular phosphate concentration on arachidonic acid production by parathyroid tissue in vitro. J Am Soc Nephrol 11:1712–1718.
Naveh-Many T, Rahamimov R, Livni N, Silver J. 1995 Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. J Clin Invest 96:1786–1793.
Denda M, Finch J, Slatopolsky E. 1996 Phosphorus accelerates the development of parathyroid hyperplasia and secondary hyperparathyroidism in rats with renal failure. Am J Kidney Dis 28:596–602.
Dusso A, Lu Y, Pavlopoulos T, Slatopolsky E. 1999 A role for enhanced expression of transforming growth factor alpha (TGF-a) in the mitogenic effects of high phosphorus on parathyroid cell growth. J Am Soc Nephrol 10:617A.
Dusso A, Naumovich L, Pavlopoulos T et al. 1998 Phosphorus regulation of parathyroid cell growth in renal failure: a role for the cyclin-dependent kinase inhibitor p21. J Am Soc Nephrol 9:564A.
Dusso AS, Pavlopoulos T, Naumovich Let al. 2001 p21(WAF1) and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth. Kidney Int 59:855–865.
Mason RS, Lissner D, Wilkinson M, Posen S. 1980 Vitamin D metabolites and their relationship to azotaemic ocsteodystrophy. Clin Endocrin 13:375–385.
Brumbaugh PF, Hughes MR, Haussler MR. 1975 Cytoplasmic and nuclear binding components for 1alpha25-dihydroxyvitamin D3 in chick parathyroid glands. Proc Natl Acad Sci U S A 72:4871–4875.
Chertow BS, Baylink DJ, Wergedal JE, Su MH, Norman AW. 1975 Decrease in serum immunoreactive parathyroid hormone in rats and in parathyroid hormone secretion in vitro by 1,25-dihydroxycholecalciferol. J Clin Invest 56:668–678.
Cantley LK, Russell J, Lettieri D, Sherwood LM. 1985 1,25-Dihydroxyvitamin D3 suppresses parathyroid hormone secretion from bovine parathyroid cells in tissue culture. Endocrinology 117:2114–2119.
Silver J, Russell J, Sherwood LM, 1985 Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci U S A 82:4270–4273.
Silver J, Naveh-Many T, Mayer H, Schmelzer HJ, Popovtzer MM. 1986 Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest 78:1296–1301.
Okazaki T, Igarashi T, Kronenberg HM. 1988 5′-flanking region of the parathyroid hormone gene mediates negative regulation by 1,25-(OH)2 vitamin D3. J Biol Chem 263: 2203–2208.
Nykjaer A, Dragun D, Walther D et al. 1999 An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96:507–515.
Kremer R, Bolivar I, Goltzman D, Hendy GN. 1989 Influence of calcium and 1,25-dihydroxycholecalciferol on proliferation and proto-oncogene expression in primary cultures of bovine parathyroid cells. Endocrinology 125:935–941.
Patel SR, Ke HQ, Vanholder R et al. 1985 Inhibition of calcitriol receptor binding to vitamin D response elements by uremic toxins. J Clin Invest 96:50–59.
Sawaya BP, Koszewski NJ, Qi Q et al. 1997 Secondary hyperparathyroidism and vitamin D receptor binding to vitamin D response elements in rats with incipient renal failure. J Am Soc Nephrol: JASN 8:271–278.
Fukuda N, Tanaka H, Tominaga Y et al. 1993 Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest 92:1436–1443.
Arnold A, Brown MF, Urena P et al. 1995 Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest 95:2047–2053.
Gogusev J, Duchambon P, Hory B et al. 1997 Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int 51:328–336.
Ritter CS, Finch JL, Slatopolsky EA, Brown AJ. 2001 Parathyroid hyperplasia in uremic rats precedes down-regulation of the calcium receptor. Kidney Int 60:1737–1744.
Wada M, Furuya Y, Sakiyama J et al. 1997 The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest 100:2977–2983.
Wada M, Nagano N, Furuya Y et al. 2000 Calcimimetic NPS R-568 prevents parathyroid hyperplasia in rats with severe secondary hyperparathyroidism. Kidney Int 57:50–58.
Felsenfeld AJ, Jara A, Pahl M et al. 1995 Differences in the dynamics of parathyroid hormone secretion in hemodialysis patients with marked secondary hyperparathyroidism. J Am Soc Nephrol 6:1371–1378.
Felsenfeld AJ, Rodriguez M, Dunlay R, Llach F. 1991 A comparison of parathyroid-gland function in haemodialysis patients with different forms of renal osteodystrophy. Nephrol Dial Transplant 6:244–251.
Goodman WG, Belin T, Gales B et al. 1995 Calcium-regulated parathyroid hormone release in patients with mild or advanced secondary hyperparathyroidism. Kidney Int 48:1553–1558.
Malberti F, Corradi B, Pagliari B et al. 1993 The sigmoidal parathyroid hormone-ionized calcium curve and the set point of calcium in hemodialysis and continuous ambulatory peritoneal dialysis. Perit Dial Int 13 Suppl 2:S476–479.
Messa P, Vallone C, Mioni G et al. 1994 Direct in vivo assessment of parathyroid hormone-calcium relationship curve in renal patients. Kidney Int 46:1713–1720.
Khosla S, Ebeling PR, Firek AF et al. 1993 Calcium infusion suggests a “set-point” abnormality of parathyroid gland function in familial benign hypercalcemia and more complex disturbances in primary hyperparathyroidism. J Clin Endocrinol Metab 76:715–720.
De Cristofaro V, Colturi C, Masa A et al. 2001 Rate dependence of acute PTH release and association between basal plasma calcium and set point of calcium-PTH curve in dialysis patients. Nephrol Dial Transplant 16:1214–1221.
Evanson JM. 1966 The response to the infusion of parathyroid extract in hypocalcaemic states. Clin Sci 31:63–75.
Somerville PJ, Kaye M. 1979 Evidence that resistance to the calcemic action of parathyroid hormone in rats with acute uremia is caused by phosphate retention. Kidney Int 16:552–560.
Massry SG, Tuma S, Dua S, Goldstein DA. 1979 Reversal of skeletal resistance to parathyroid hormone in uremia by vitamin D metabolites: evidence for the requirement of 1,25(OH)2D3 and 24,25(OH)2D3. J Lab Clin Med 94:152–157.
Somerville PJ, Kaye M. 1978 Resistance to parathyroid hormone in renal failure: role of vitamin D metabolites. Kidney Int 14:245–254.
Galceran T, Martin KJ, Morrissey JJ, Slatopolsky E. 1987 Role of 1,25-dihydroxyvitamin D on the skeletal resistance to parathyroid hormone. Kidney Int 32:801–807.
Olgaard K, Arbelaez M, Schwartz J et al. 1982 Abnormal skeletal response to parathyroid hormone in dogs with chronic uremia. Calcif Tissue Int 34:403–407.
Olgaard K, Schwartz J, Finco D et al. 1982 Extraction of parathyroid hormone and release of adenosine 3′,5′-monophosphate by isolated perfused bones obtained from dogs with acute uremia. Endocrinology 111:1678–1682.
Picton ML, Moore PR, Mawer EB et al. 2000 Down-regulation of human osteoblast PTH/PTHrP receptor mRNA in end- stage renal failure. Kidney Int 58:1440–1449.
Nguyen-Yamamoto L, Rousseau L, Brossard JH et al. 2001 Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the PTH/PTH-related peptide receptor. Endocrinology 142:1386–1392.
Slatopolsky E, Finch J, Clay P et al. 2000 A novel mechanism for skeletal resistance in uremia. Kidney Int 58:753–761.
Di Stefano A, Roinel N, de Rouffignac C, Wittner M. 1993 Transepithelial Ca2+ and Mg2+ transport in the cortical thick ascending limb of Henle’s loop of the mouse is a voltage-dependent process. Renal Physiol Biochem 16:157–166.
Divieti P, Inomata N, Chapin K et al. 2001 Receptors for the carboxyl-terminal region of PTH(1-84) are highly expressed in osteocytic cells. Endocrinology 142:916–925.
Divieti P, John MR, Juppner H, Bringhurst FR. 2002 Human PTH-(7-84) inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology 143:171–176.
González E, Martin K. 1992 Aluminum and renal osteodystrophy: a diminishing clinical problem. Trends Endocrinol Metab 3:371–375.
Torres A, Lorenzo V, Hernandez D et al. 1995 Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int 47:1434–1442.
Hernandez D, Concepcion MT, Lorenzo V et al. 1994 Adynamic bone disease with negative aluminium staining in predialysis patients: prevalence and evolution after maintenance dialysis. Nephrol Dial Transplant 9:517–523.
Hutchison AJ, Whitehouse RW, Boulton HF et al. 1993 Correlation of bone histology with parathyroid hormone, vitamin D3, and radiology in end-stage renal disease. Kidney Int 44:1071–1077.
Couttenye MM, D’Haese PC Verschoren WJ et al. 1999 Low bone turnover in patients with renal failure. Kidney Int 56:S70–76.
Kraut JA. 1995 The role of metabolic acidosis in the pathogenesis of renal osteodystrophy. Adv Ren Replace Ther 2:40–51.
Kleeman CR. 1994 The role of chronic anion gap and/or nonanion gap acidosis in the osteodystrophy of chronic renal failure in the predialysis era: a minority report. Miner Electrolyte Metab 20:81–96.
Bushinsky DA. 1995 The contribution of acidosis to renal osteodystrophy. Kidney Int 47:1816–1832.
Frick KK, Jiang L, Bushinsky DA. 1997 Acute metabolic acidosis inhibits the induction of osteoblastic egr-1 and type 1 collagen. Am J Physiol 272:C1450–1456.
Frick KK, Bushinsky DA. 1998 Chronic metabolic acidosis reversibly inhibits extracellular matrix gene expression in mouse osteoblasts. Am J Physiol 275:F840–847.
Frick KK, Bushinsky DA. 1999 In vitro metabolic and respiratory acidosis selectively inhibit osteoblastic matrix gene expression. Am J Physiol 277:F750–755.
Frick KK, Bushinsky DA. 2002 Metabolic acidosis stimulates expression of rank ligand RNA. J Am Soc Nephrol 13:576A.
Bushinsky DA. 1995 Stimulated osteoclastic and suppressed osteoblastic activity in metabolic but not respiratory acidosis. Am J Physiol 268:C80–88.
Cunningham J, Bikle DD, Avioli LV. 1984 Acute, but not chronic, metabolic acidosis disturbs 25-hydroxyvitamin D3 metabolism. Kidney Int 25:47–52.
Disthabanchong S, Hassan H, McConkey CL et al. 2004 Regulation of PTH1 receptor expression by uremic ultrafiltrate in UMR 106-01 osteoblast-like cells. Kidney Int 65:897–903.
Lefebvre A, de Vernejoul MC, Gueris J et al. 1989 Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney Int 36:1112–1118.
Manolagas SC, Weinstein RS. 1999 New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res 14:1061–1066.
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. 1998 Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282.
Weinstein RS, Nicholas RW, Manolagas SC. 2000 Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab 85:2907–2912.
Weinstein RS, Chen JR, Powers CC et al. 2002 Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest 109:1041–1048.
Disthabanchong S, Gonzalez EA. 2001 Regulation of bone cell development and function: implication for renal osteodystrophy. J Invest Med 49:240–249.
Hruska K. 1998 New concepts in renal osteodystrophy. Nephrol Dial Transpl 13:2755–2760.
Monier-Faugere MC, Malluche HH. 1997 Role of cytokines in renal osteodystrophy. Curr Opin Nephrol Hypertens 6:327–332.
Andress DL, Pandian MR, Endres DB, Kopp JB. 1989 Plasma insulin-like growth factors and bone formation in uremic hyperparathyroidism. Kidney Int 36:471–477.
Andress DL, Birnbaum RS. 1992 Human osteoblastderived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J Biol Chem 267:22,467–22,472.
Gonzalez EA, Lund RJ, Martin KJ et al. 2002 Treatment of a murine model of high-turnover renal osteodystrophy by exogenous BMP-7. Kidney Int 61:1322–1331.
Davies MR, Lund RJ, Mathew S, Hruska KA. 2005 Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol 16:917–928.
Kimmel PL, Phillips TM, Simmens SJ et al. 1998 Immunologic function and survival in hemodialysis patients. Kidney Int 54:236–244.
Herbelin A, Nguyen AT, Zingraff J et al. 1990 Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int 37:116–125.
Herbelin A, Ureña P, Nguyen AT et al. 1991 Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int 39:954–960.
Ferreira A. 1998 Biochemical markers of bone turnover in the diagnosis of renal osteodystrophy: what do we have, what do we need? Nephrol Dial Transpl 13 Suppl 3:29–32.
Nakagawa N, Kinosaki M, Yamaguchi K et al. 1998 RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun 253:395–400.
Montalbán C, García-Unzueta MT, De Francisco AL, Amado JA. 1999 Serum interleukin-6 in renal osteodystrophy: relationship with serum PTH and bone remodeling markers. Horm Metab Res 31:14–17.
Kazama JJ, Shigematsu T, Yano K et al. 2002 Increased circulating levels of osteoclastogenesis inhibitory factor (osteoprotegerin) in patients with chronic renal failure. Am J Kidney Dis 39:525–532.
Haas M, Leko-Mohr Z, Roschger P et al. 2002 Osteoprotegerin and parathyroid hormone as markers of high-turnover osteodystrophy and decreased bone mineralization in hemodialysis patients. Am J Kidney Dis 39:580–586.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin, K.J., González, E.A. Pathophysiology of renal osteodystrophy. Clinic Rev Bone Miner Metab 5, 11–19 (2007). https://doi.org/10.1007/BF02736667
Issue Date:
DOI: https://doi.org/10.1007/BF02736667