Summary
By means of new definitions of separable system, separable state and energetic process, the first postulate of thermodynamics and the definition of energy are rigorously extended to nonsimple systems. The definition of energy for open systems presented in a previous paper (Zanchini,Nuovo Cimento B,101, 453 (1988)) is simplified. Then, the statement of the second postulate proposed by the MIT school of thermodynamics is stated in a more precise form, which does not present the undefined concept of parameters. As an application of the logical scheme so obtained, the Gibbs equations for systems contained in electric or magnetic fields are deduced rigorously.
Similar content being viewed by others
References
E. Zanchini:Nuovo Cimento B,101, 453 (1988).
E. Fermi:Thermodynamics (Prentice-Hall, Englewood Cliffs, N.J., 1937).
A. B. Pippard:Classical Thermodynamics (Cambridge University Press, Cambridge, 1957).
P. T. Landsberg:Thermodynamics with Quantum Statistical Illustrations (Interscience Publishers, New York, N.Y., London, 1961).
G. N. Hatsopoulos andJ. H. Keenan:Principles of General Thermodynamics (Wiley, New York, N. Y., 1965).
N. W. Zemansky:Heat and Thermodynamics (McGraw-Hill, New York, N.Y., 1968).
J. H. Keenan, G. N. Hatsopoulos andE. P. Gyftopoulos:Encyclopaedia Britannica,18, 290 (1981).
G. P. Beretta:Termodinamica Generale (C.N.R.-P.F.E., Roma, 1982).
E. Zanchini:Found. Phys.,16, 923 (1986).
E. P. Gyftopoulos andG. P. Beretta:Invited Paper at the 1987 ASME Winter Annual Meeting, Boston, Mass., 1987.
H. B. Callen:Thermodynamics (Wiley, New York, N.Y., 1960).
L. C. Woods:The Thermodynamics of Fluid Systems (Oxford University Press, 1975).
H. B. Callen:Thermodynamics and an Introduction to Thermostatistics (Wiley, New York, N. Y., 1985).
See in particular ref. [8], and the firts part of the statement proposed in ref. [10]. The second part of the latter statement introduces a strong hypothesis of interconnectability by «reversible weight processes» which should be carefully analysed, and that becomes unnecessary if the definition of energy for open systems proposed in ref. [1] and in this paper is adopted.
L. D. Landau andE. M. Lifshitz:Electrodynamics of Continuous Media (Pergamon Press, Oxford, 1960).
For rigorous definitions of microscopic and macroscopic distributions of charge density and current density, see for exampleJ. D. Jackson:Calssical Electrodynamics (Wiley, New York, N.Y., 1975).
The definition of simple system proposed in this paper is broader than those presented in ref.[5]. 7J. H. Keenan, G. N. Hatsopoulos andE. P. Gyftopoulos:Encyclopaedia Britannica,18, 290 (1981). 8G. P. Beretta:Termodinamica Generale (C.N.R.-P.F.E., Roma, 1982). 11H. B. Callen:Thermodynamics (Wiley, New York, N.Y., 1960). 13H. B. Callen:Thermodynamics and an Introduction to Thermostatistics (Wiley, New. York, N.Y., 1985). In fact, according to the definition proposed here, the fundamental relation of a simple system can contain several parameters, and not only volumeV. See, for example, the fundamental relation for a homogeneously strained elastic system presented in ref. [11]H. B. Callen:Thermodynamics (Wiley, New York, N. Y., 1960).
In ref. [1]. hypothesis 1 was automatically satisfied by the definition of a set of separable states.
If only states of macroscopic systems are considered, then each compositionn is a vector of mole numbers. We can choose asN 0 the state in which the external field is zero, different particles are in separated and noninteracting boxes, and each box contains particles in the stable equilibrium state {T 0,v 0}:T 0=reference temperature,v 0=reference molar volume. Hypothesis 2 is satisfied if, for each kind of particle, the reference state forr moles is the union ofm reference states withr/m moles, or can be interconnected with this state by a zero-work reversible energetic process, for every positive integer numberm. This condition can be considered as satisfied, in normal applications.
E. Zanchini:I principi basilari della termodinamica (C.U.S.L., Bologna, 1987).
We will call centre of a region of spaceR the centre of mass of any system with uniform mass density contained inR.
The fieldE e (z) can be obtained by adjoining capacitors, which form two infinitely wide parallel plates such that the electric-charge density in each plate is a step function ofz, with infinitesimal steps.
J. A. Stratton:Electromagnetic Theory (McGraw-Hill, New York, N. Y., 1941).
The second integral in (6.5) refers to the process of formation of the field. If this process is a reversible and energetic process ofA+C, as it is considered here, thenW e represents the change of energy ofA+C. If this process is a reversible and energetic process of a systemA+C+R, whereR is a heat reservoir, and if the temperatureT ofA is constant and equals the temperatureT 0 ofR, thenW e represents the change of the Helmoltz free energy ofA++C. The latter interpretation is clarified, for instance, byR. Becker, inTeoria dell’elettricità (Sansoni Edizioni Scientifiche, 1949).
The fieldH e(x) can be obtained by an infinitely long solenoid alongx, such that the electric current in the coil is a step function ofx, with infinitesimal steps.
It is possible to prove that the work done on systemA in any reversible energetic process ofA with constantV andJ equals the decrease of the following state functional of
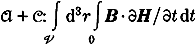 , where the time integral refers to any reversible energetic process ofA+C, withR=constant, in which the macroscopic current distribution onC goes from 0 toJ andA reaches the state under examination. A similar result, used in ref.[15]L. D. Landau andE. M. Lifshitz:Electrodynamics of Continuous Media (Pergamon Press, Oxford, 1960). holds for a reversible isothermal process ofA with constantV andJ.
, where the time integral refers to any reversible energetic process ofA+C, withR=constant, in which the macroscopic current distribution onC goes from 0 toJ andA reaches the state under examination. A similar result, used in ref.[15]L. D. Landau andE. M. Lifshitz:Electrodynamics of Continuous Media (Pergamon Press, Oxford, 1960). holds for a reversible isothermal process ofA with constantV andJ.
Author information
Authors and Affiliations
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/BF02728573.
Rights and permissions
About this article
Cite this article
Zanchini, E. Thermodynamics: Energy of nonsimple systems and second postulate. Nuov Cim B 107, 123–139 (1992). https://doi.org/10.1007/BF02722911
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02722911




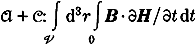 , where the time integral refers to any reversible energetic process ofA+C, withR=constant, in which the macroscopic current distribution onC goes from 0 toJ andA reaches the state under examination. A similar result, used in ref.[15]
, where the time integral refers to any reversible energetic process ofA+C, withR=constant, in which the macroscopic current distribution onC goes from 0 toJ andA reaches the state under examination. A similar result, used in ref.[15]