Abstract
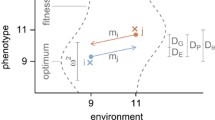
The relative contributions of ancestry, chance, and past and ongoing election to variation in one adaptive (larval feeding rate) and one seemingly nonadaptive (pupation height) trait were determined in populations ofDrosophila melanogaster adapting to either low or high larval densities in the laboratory. Larval feeding rates increased rapidly in response to high density, and the effects of ancestry, past selection and chance were ameliorated by ongoing selection within 15–20 generations. Similarly, in populations previously kept at high larval density, and then switched to low larval density, the decline of larval feeding rate to ancestral levels was rapid (15-20 generations) and complete, providing support for a previously stated hypothesis regarding the costs of faster feeding inDrosophila larvae. Variation among individuals was the major contributor to variation in pupation height, a trait that would superficially appear to be nonadaptive in the environmental context of the populations used in this study because it did not diverge between sets of populations kept at low versus high larval density for many generations. However, the degree of divergence among populations (FST) for pupation height was significantly less than expected for a selectively neutral trait, and we integrate results from previous studies to suggest that the variation for pupation height among populations is constrained by stabilizing selection, with a flat, plateau-like fitness function that, consequently, allows for substantial phenotypic variation within populations. Our results support the view that the genetic imprints of history (ancestry and past selection) in outbreeding sexual populations are typically likely to be transient in the face of ongoing selection and recombination. The results also illustrate the heuristic point that different forms of selection-for example directional versus stabilizing selection—acting on a trait in different populations may often not be due to differently shaped fitness functions, but rather due to differences in how the fitness function maps onto the actual distribution of phenotypes in a given population. We discuss these results in the light of previous work on reverse evolution, and the role of ancestry, chance, and past and ongoing selection in adaptive evolution.
Similar content being viewed by others
References
Bauer S. J. and Sokolowski M. B. 1985 A genetic analysis of path length and pupation height in a natural population ofDrosophila melanogaster.Can. J. Genet. Cytol. 27, 334–340.
Bürger R. and Gimelfarb A. 1999 Genetic variation maintained in multilocus models of additive quantitative traits under stabilizing selection.Genetics 152, 807–820.
Burnet B., Sewell D. and Bos M. 1977 Genetic analysis of larval feeding behaviour inDrosophila melanogaster. II. Growth relations and competition between selected lines.Genet. Res. 30, 149–161.
Chippindale A. K., Alipaz J. A., Chen H. W. and Rose M. R. 1997 Experimental evolution of accelerated development inDrosophila. 1. Developmental speed and larval survival.Evolution 51, 1536–1551.
Cohan F. M. and Hoffmann A. A. 1989 Uniform selection as a diversifying force in evolution: evidence fromDrosophila.Am. Nat. 134, 613–637.
Falconer D. S. 1981Introduction to quantitative genetics, 2nd edition. Longman, London.
Gimelfarb A. 1989 Genotypic variance for a quantitative character maintained under stabilizing selection without mutations: epistasis.Genetics 123, 217–227.
Gould S. J. and Lewontin R. C. 1979 The spandrels of San Marco and the Panglossian paradigm. A critique of the adaptationist programme.Proc. R. Soc. London. B205, 581–598.
Guo P.-Z., Mueller L. D. and Ayala F. J. 1991 Evolution of behaviour by density-dependent natural selection.Proc. Natl. Acad. Sci. USA 88, 10905–10906.
Hartl D. L. and Clark A. G. 1989Principles of population genetics, 2nd edition. Sinauer, Sunderland.
Ives P. T. 1970 Further studies of the South Amherst population ofDrosophila melanogaster.Evolution 24, 507–518.
Joshi A. and Mueller L. D. 1988 Evolution of higher feeding rate inDrosophila due to density-dependent natural selection.Evolution 42, 1090–1092.
Joshi A. and Mueller L. D. 1993 Directional and stabilizing density-dependent natural selection for pupation height inDrosophila melanogaster.Evolution 47, 176–184.
Joshi A. and Mueller L. D. 1996 Density-dependent natural selection in Drosophila: trade-offs between larval food acquisition and utilization.Evol. Ecol. 10, 463–474.
Joshi A. and Thompson J. N. 1995 Alternative routes to the evolution of competitive ability in two competing species ofDrosophila.Evolution 49, 616–625.
Joshi A. and Thompson J. N. 1996 Evolution of broad and specific competitive ability in novel versus familiar environments inDrosophila species.Evolution 50, 188–194.
Joshi A. and Thompson J. N. 1997 Adaptation and specialization in a two-resource environment inDrosophila species.Evolution 51, 846–855.
Kimura M. 1968 Evolutionary rate at the molecular level.Nature 217, 624–626.
King J. L. and Jukes T. L. 1969 Non-Darwinan evolution.Science 164, 788–798.
Leips J. and Mackay T. F. C. 2000 Quantitative trait loci for life span inDrosophila melanogaster: interactions with genetic background and larval density.Genetics 155, 1773–1788.
Lenski R. E. and Travisano M. 1994 Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations.Proc. Natl. Acad. Sci. USA 91, 6808–6814.
Lloyd E. A. and Gould S. J. 1993 Species selection on variability.Proc. Natl. Acad. Sci. USA 90, 595–599.
Long A. D. and Singh R. S. 1995 Molecules versus morphology: the detection of selection acting on morphological characters along a cline inDrosophila melanogaster.Heredity 74, 569–581.
Markow T. A. 1979 A survey of intra- and inter-specific variation for pupation height inDrosophila. Behav.Genet. 9, 209–217.
Mayr E. 1983 How to carry out the adaptationist program?Am. Nat. 121, 324–334.
Mueller L. D. 1990 Density-dependent natural selection does not increase efficiency.Evol. Ecol. 4, 290–297.
Mueller L. D. 1995 Adaptation and density-dependent natural selection. InGenetics of natural populations: the continuing importance of Theodosius Dobzhansky (ed. L. Levine), pp. 101–124. Columbia University Press, New York.
Mueller L. D. and Sweet V. F. 1986 Density-dependent natural selection in Drosophila: evolution of pupation height.Evolution 40, 1354–1356.
Mueller L. D., Guo P. Z. and Ayala F. J. 1991 Density-dependent natural selection and trade-offs in life history traits.Science 253, 433–435.
Mueller L. D., Graves J. L. and Rose M. R. 1993 Interactions between density-dependent and age-specific selection inDrosophila melanogaster.Funct. Ecol. 7, 469–479.
Mueller L. D., Joshi A. and Borash D. J. 2000 Does population stability evolve?Ecology 81, 1273–1285.
Neter J., Wasserman W. and Kutner M. H. 1990Applied linear statistical models: regression, analysis of variance, and experimental design, 3rd edition. Irwin, Boston.
Parker G. A. and Maynard Smith J. 1990 Optimality theory in evolutionary biology.Nature 348, 27–33.
Pletcher S. D., Macdonald S. J., Marguerie R., Certa U., Stearns S. C. and Partridge L. 2002 Genome-wide transcript profiles in aging and calorically restrictedDrosophila melanogaster.Curr. Biol. 12, 712–723.
Prasad N. G. and Joshi A. 2003 What have two decades of laboratory life-history evolution studies onDrosophila melanogaster taught us?J. Genet. 82, 45–76.
Prasad N. G., Shakarad M., Anitha D., Rajamani M. and Joshi A. 2001 Correlated responses to selection for faster development and early reproduction in Drosophila: the evolution of larval traits.Evolution 55, 1363–1372.
Prout T. and Barker J. S. F. 1993F statistics inDrosophila buzzatii: selection, population size and inbreeding.Genetics 134, 369–375.
Rainey P. B. and Travisano M. 1998 Adaptive radiation in a heterogeneous environment.Nature 394, 69–72.
Rose M. R. 1982 Antagonistic pleiotropy, dominance, and genetic variation.Heredity 48, 63–78.
Rose M. R. 1984 Laboratory evolution of postponed senescence inDrosophila melanogaster.Evolution 38, 1004–1010.
Rose M. R., Graves J. L. and Hutchinson E. W. 1990 The use of selection to probe patterns of pleiotropy in fitness characters. InGenetics, evolution and coordination of insect life histories (ed. F. Gilbert), pp. 29–41. Springer, New York.
Sameoto D. D. and Miller R. S. 1968 Selection of pupation site byDrosophila melanogaster andD. simulans.Ecology 49, 177–180.
Sewell D., Burnet B. and Conolly K. 1975 Genetic analysis of larval feeding behaviour inDrosophila melanogaster.Genet. Res. 24, 163–173.
Sokolowski M. B. 1980 Foraging strategies ofDrosophila melanogaster: a chromosomal analysis.Behav. Genet. 10, 291–302.
Sokolowski M. B. and Bauer S. J. 1989 Genetic analyses of pupation distance inDrosophila melanogaster.Heredity 62, 177–183.
Teótonio H. and Rose M. R. 2000 Variation in the reversibility of evolution.Nature 408, 463–466.
Teótonio H. and Rose M. R. 2001 Perspective: reverse evolution.Evolution 55, 653–660.
Teótonio H., Matos M. and Rose M. R. 2002 Reverse evolution of fitness inDrosophila melanogaster.J. Evol. Biol. 15, 608–617.
Thompson J. N. 1994The coevolutionary process. University of Chicago Press, Chicago.
Travisano M. and Lenski R. E. 1996 Long-term experimental evolution inEscherichia coli. IV. Targets of selection and the specificity of adaptation.Genetics 143, 15–26.
Travisano M., Mongold J. A., Bennett A. F. and Lenski R. E. 1995 Experimental tests of the roles of adaptation, chance, and history in evolution.Science 267, 87–90.
Vieira C., Pasyukova E. G., Zeng Z. B., Hackett J. B., Lyman R. F. and Mackay T. F. C. 2000 Genotype-environment interaction for quantitative trait loci affecting life span inDrosophila melanogaster.Genetics 154, 213–227.
Wade M. J. and Kalisz S. 1990 The causes of natural selection.Evolution 44, 1947–1955.
White K. P., Rifkin S. A., Hurban P. and Hogness D. S. 1999 Microarray analysis ofDrosophila development during metamorphosis.Science 286, 2179–2184.
Williams G. C. 1992Natural selection: domain, levels and challenges. Oxford University Press, Oxford.
Wright S. 1951 The genetic structure of populations.Ann. Eugen. 15, 323–354.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, A., Castillo, R.B. & Mueller, L.D. The contribution of ancestry, chance, and past and ongoing selection to adaptive evolution. J Genet 82, 147–162 (2003). https://doi.org/10.1007/BF02715815
Issue Date:
DOI: https://doi.org/10.1007/BF02715815