Abstract
Introduction
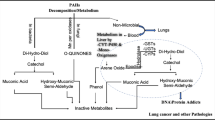
Polycyclic aromatic hydrocarbons (PAH) are environmental contaminants that have been of interest in cancer research for a considerable length of time. DNA adduct formation is considered a marker and indicator for exposure to PAH. The aim of this study was to determine PAH-DNA adduct levels in peripheral blood lymphocytes and urine obtained from workers exposed to PAH, and to evaluate tobacco use, GSTM1 and GSTT1 as possible contributory risk factors.
Material and methods
Our study included a random sample of 66 workers exposed to PAH and 49 non-exposed workers.
Results
PAH-DNA adduct levels of exposed workers were lower than that of the non-exposed group (p<0.05). However, current smoking, GSTM1-negatives, and current smoking with GSTM1-negatives were more frequent in the non-exposed group. In addition, non-exposed workers reported exposure to PAH in their current jobs, as compared with the exposed group (p<0.001). Linear regression analysis identified the levels of benzo-[b]-fluoranthene in the working area as the only significant DNA adduct-forming risk factor (p=0.025).
Conclusion
Further research, with an appropriately large sample size, is highly recommended in measuring PAH-DNA adduct levels and evaluating their relationship with the different types of PAH.
Resumen
|Introducción
Los hidrocarburos aromáticos policíclicos (HAP) representan un contaminante ambiental de gran interés en la investigación del cáncer. La formación de aductos de ADN está considerada como un indicador de la exposición a los HAP. El objetivo de este estudio es determinar los niveles de aductos de HAP-ADN en linfocitos sanguíneos y orina en trabajadores expuestos a los HAP, y evaluar sus relaciones con el tabaco, la GSTM1 y la GSTT1 como posibles factores de riesgo.
|Material y métodos
Nuestro estudio incluyó una muestra aleatoria de 66 trabajadores expuestos y 49 no expuestos a los HAP.
|Resultados
Los niveles de aductos de HAP-ADN en los trabajadores expuestos fueron inferiores a los no expuestos (p<0,05). Sin embargo, dentro del grupo de los trabajadores expuestos había más fumadores actuales, GSTM1 negativos y fumadores actuales con GSTM1 negativos. Además, los trabajadores no expuestos informaron exposiciones a los HAP en trabajos en comparación con el grupo de los expuestos (p<0,001). El análisis por regresión lineal identificó los niveles del benzo (b) fluorantene en las áreas de trabajo como el único factor de riesgo significativo para los aductos de ADN (p=0,025).
|Conclusiones
Son recomendables futuros estudios con un tamaño de muestra suficiente para evaluar los niveles de aductos de HAP-ADN y sus relaciones con los diferentes tipos de HAP.
Similar content being viewed by others
References
Georgiadis P, Topinka J, Stoikidou M, et al. Biomarkers of genotoxicity of air pollution (the AULIS project): Bulky DNA adducts in subjects with moderate to low exposures to airborne polycyclic aromatic hydrocarbons and their relationship to environmental tobacco smoke and other parameters. Carcinogenesis 2001;22(9):1447–57.
Yu H. Environmental carcinogenic polycyclic aromatic hydrocarbons: photochemistry and phototoxicity. J Environ Sci Health (Part C) Environ Carcinog Ecotoxicol Rev 2002;20(2):149–83.
Godschalk RW, Schooten FJ, Bartsch H. A critical evaluation of DNA adducts as biological markers for human exposure to polycyclic aromatic compounds. J Biochem Mol Biol 2003;36(1):1–11.
Palli D, Russo A, Masala G, et al. DNA adduct levels and DNA repair polymorphisms in traffic-exposed workers and a general population sample. Int J Cancer 2001;94(1):121–7.
Peluso M, Ceppi M, Munnia A, Puntoni R, Parodi S. Analysis of 1332P-DNA postlabeling studies on occupational cohorts exposed to air pollution. Am J Epidemiol 2001;153(6):546–58.
Akkineni LK, Zeisig M, Baranczewski P, Ekstrom L-G, Moller L. Formation of DNA adducts from oil-derived products analyzed by32P-HPLC. Arch Toxicol 2001; 74:720–31.
Jongeneelen FJ, Anzion RBM, Scheepers PTJ, et al. 1-hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann Occup Hyg 1988;32:35–43.
Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis 2002;23(12):1979–2004.
Pavanello S, Favretto D, Brugnone F, Mastrangelo G, Dal Pra G, Clonfero E. HPLC/fluorescence determination of anti-PBDE-DNA adducts in mononuclear white blood cells from PAH-exposed humans. Carcinogenesis 1999;20(3):431–5.
Rojas M, Cascorbi I, Alexandrov K, et al. Modulation of benzo[a]pyrene diolepoxide-DNA adduct levels in human white blood cells by CYP1A1, GSTM1 and GSTT1 polymorphism. Carcinogenesis 2000;21(1):35–41.
Pavanello S, Clonfero E. Biomarkers of gentotoxic risk and metabolic polymorphism. Med Lav 2000;91(5):431–69.
Alexandrov K, Cascorbi I, Rojas M, Bouvier G, Kriek E, Bartsch H. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers' lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis 2002;23(12):1969–77.
Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers Prev 2000; 9(1):3–28.
Soni M, Madurantakan M, Krishnaswamy K. Glutathione S-transferase Mu (GST Mu) deficiency and DNA adducts in lymphocytes of smokers. Toxicology 1998; 126:155–62.
Gupta RC, Reddy MV, Randerath K.32P-postlabeling analysis of non-radioactive aromatic carcinogen-DNA adducts. Carcinogenesis 1982;3(9):1081–92.
Oude Ophuis MB, Van Lieshout EM, Roelofs HM, Peters WH, Manni JJ. Glutathione S-transferase M1 and T1 and cytochrome P450 1A1 polymorphisms in relation to the risk for benign and malignant head and neck lesions. Cancer 1998;82:936–43.
Jongeneelen FJ, Anzion RBM, Henderson PTh. Determination of hydroxylated polycyclic aromatic hydrocarbons in urine. J Chromatogr 1987;413:227–32.
NIOSH. Polycyclic aromatic hydrocarbons. Manual of analytical methods. En: Eller PM, editor. 3rd ed. Volume 2. Cincinnati, Ohio State: 1984;p. 84–100.
Poirier MC, Santella RM, Weston A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis 2000;21(3):353–9.
Schut HAJ, Shiverick KT. DNA adducts in humans as dosimeters of exposure to environmental, occupational, or dietary genotoxins. FASEB J 1992;6:2942–51.
Beach AC, Gupta RC. Human biomonitoring and the32P-postlabeling assay. Carcinogenesis 1992;13:1053–74.
Randerath K, Randerath E.32P-postlabeling methods for DNA adduct detection: Overview and critical evaluation. Drug Metabol Rev 1994;26:67–85.
Phillips DH. Detection of DNA modification by32P-postlabeling assay. Mutat Res 1997;378:1–12.
Kriek E, Rojas M, Alexandrov K, Bartsch H. Polycyclic aromatic hydrocarbon-DNA adducts in humans: relevance as biomarkers for exposure and cancer risk. Mutat Res 1998;400(1–2):215–31.
Brescia G, Celotti L, Clonfero E, et al. Influence of cytochrome P450 1A1 and glutathione S-transferase genotypes on biomarker levels in coke oven workers. Arch Toxicol 1999;73(8–9):431–9.
Abdel-Rahman AG, Allam MF, Mansour MT, Mustafa MA-A. PAH-DNA adducts in a petrol refinery in Egypt. Eur J Cancer Prev 2001;10:469–72.
Vodička P, Tvrdik T, Osterman-Golkar S, et al. An evaluation of styrene genotoxicity using several biomarkers in a 3-year follow-up study of hand-lamination workers. Mutat Res 1999;445:205–24.
Vineis P, Caporaso N. Tobacco and cancer: epidemiology and the laboratory. Environ Health Perspect 1995;103(2):156–60.
Butkiewicz D, Grzybowska E, Hemminki K, et al. Modulation of DNA adduct levels in human mononuclear white blood cells and granulocytes by CYP1A1, CYP2D6 and GSTM1 genetic polymorphisms. Mutat Res 1998;415:97–108.
Pavanello S, Gabbani G, Mastrangelo G, Brugnone F, Maccacaro G, Clonfero E. Influence of GSTM1 genotypes on anti-BPDE-DNA adduct levels in mononuclear white blood cells of humans exposed to PAH. Int Arch Occup Environ Health 1999;72(4):238–46.
Pavanello S, Clonfero E. Biological indicators of genotoxic risk and metabolic polymorphisms. Mutat Res 2000; 463(3):285–308.
Kuljukka T, Savela K, Vaaranrinta R, et al. Low response in white blood cell DNA adducts among workers in a highly polluted cokery environment. JOEM 1998;40(6):529–37.
Carstensen U, Hou S-M, Alexandrie A-K, et al. Influence of genetic polymorphisms of biotransformation enzymes on gene mutations, strand breaks of deoxyribonucleic acid, micronuclei in mononuclear blood cells and urinary 8-hydroxydeoxyguanosine in potroom workers exposed to polyaromatic hydrocarbons. Scand J Work Environ Health 1999;25(4):351–60.
Van Delft JH, Steenwinkel MS, van Asten JG, et al. Biological monitoring the exposure to polycyclic aromatic hydrocarbons of coke oven workers in relation to smoking and genetic polymorphisms for GSTM1 and GSTT1. Ann Occup Hyg 2001;45(5):395–408.
Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg 2001;45(1):3–31.
Pan G, Hanaoka T, Yamano Y, et al. A study of multiple biomarkers in coke oven workers- a cross-sectional study in China. Carcinogenesis 1998;19 (11):1963–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Rahman, A.G., Allam, M.F., El Gaafary, M.M. et al. Assessment of carcinogenicity, using PAH-DNA adducts, in workers exposed to polycyclic aromatic hydrocarbons. Rev Oncol 6, 159–164 (2004). https://doi.org/10.1007/BF02710117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02710117