Abstract
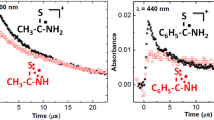
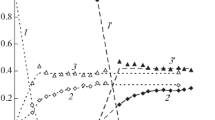
In neutral aqueous solution of (phenylthio)acetic acid, hydroxyl radical is observed to react with a bimolecular rate constant of 7.2 × 10-1 dm3 mol−s− and the transient absorption bands are assigned to•OH radical addition to benzene and sulphur with a rough estimated values of 50 and 40% respectively. The reaction of the•OH radical with diphenyl sulphide (k = 4.3 × 108 dm3 mol−1 s−1) is observed to take place with formation of solute radical cation, OH-adduct at sulphur and benzene with estimated values of about 12, 28 and 60% respectively. The transient absorption bands observed on reaction of•OH radical, in neutral aqueous solution of 4-(methylthio)phenyl acetic acid, are assigned to solute radical cation (λmax = 550 and 730 nm), OH-adduct at sulphur (λmax = 360 nm) and addition at benzene ring (λmax = 320 nm). The fraction of•OH radical reacting to form solute radical cation is observed to depend on the electron-withdrawing power of substituted group. In acidic solutions, depending on the concentration of acid and electron-withdrawing power, solute radical cation is the only transient species formed on reaction of•OH radical with the sulphides studied.
Similar content being viewed by others
References
Glass R S 1970 InSulphur centred reactive intermediates in chemistry and biology (eds) C Chatgilialogu and K-D Asmus (New York: Plenum) vol. 197, p. 213
Asmus K-D and Bonifacic M 1999 InS-Centred radicals (ed.) Z B Alfassi (New York: John Wiley and Sons) p. 141
Wardman P 1988 InGlutathiene conjugation (eds) H Sies and B Ketterer (New York: Academic Press) p. 43
von Sonntag C 1987 InThe chemical basis of radiation biology (New York: Taylor and Francis) p. 31
Mariano P S and Stavinoha J L 1984 InSynthetic organic photochemistry (ed.) W M Horspoel (New York: Plenum) p. 84
Lewis F D 1986 InPhotoinduced electron transfer (eds) M A Fox and M Chanon (Amsterdam: Elsevier)
Torchinsky Yu M 1979 InSulphur in proteins (ed.) D Metzer (Oxford: Pergamon)
Mőnig M, Gőbl and Asmus K-D 1985J. Chem. Soc., Perkin Trans. 2 647
Gőbl M and Asmus K-D 1984J. Chem. Soc., Perkin Trans. 2 691
Schőneich C and Bobrowski K 1994J. Phys. Chem. 98 12613
Maity D K, Mohan H and Mittal J P 1994J. Chem. Soc., Faraday Trans. 90 703
Gawandi V B, Mohan H and Mittal J P 1999J. Chem. Soc., Perkin Trans. 2 1425
Deng Y, Illies A J, James M A, McKee M L and Peschke M 1995J. Phys. Chem. 117 420
Iole M, Steenken S and Baciocchi F 1997J. Phys. Chem. 101 2979
Engman L, Lind J and Merenyi G 1994J. Phys. Chem. 98 3174
Yagci Y, Schnabel W, Wilpert A and Bendig J 1994J. Chem. Soc., Faraday Trans. 90 287
Guha S N, Moorthy P N, Kishore K and Rao K N 1987Proc. Indian Acad. Sci. (Chem. Sci.) 99 261
Chaudhri S A, Mohan H, Anklam E and Asmus K-D 1996J. Chem. Soc., Perkin Trans. 2 383
Taft W 1957J. Chem. Phys. 26 93
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohan, H. Effect of electron-withdrawing power of the substituted group on•OH radical reaction with substituted aryl sulphides: A pulse radiolysis study. J Chem Sci 114, 749–758 (2002). https://doi.org/10.1007/BF02708868
Issue Date:
DOI: https://doi.org/10.1007/BF02708868