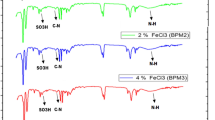
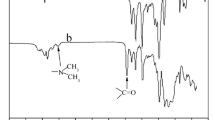
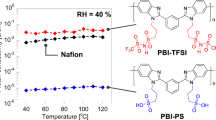
Abstract
Bipolar membranes (BPMs) were prepared by using commercial ion exchange membranes and hydrophilic polymer as a binder to investigate the effects of the interface hydrophilicity on water-splitting capacity. In this study, polyHEMA/MPD cross-linked with TMC was used as a binding material to enhance the BPM interface hydrophilicity. The enhanced hydrophilicity of the BPM interface accelerated the water-splitting reaction because the hydrophilic polymer layer increases the water activity by attracting water from the ion exchange layers to the space charge region. In addition, a mechanism of the metal catalytic reaction was proposed. Metal species were immobilized in the BPM in a hydroxide form and possibly react with water molecules and the quaternary ammonium groups reversibly. It was also observed that metal species immobilized in the membrane improved the water-splitting efficiency by increment of the membrane wetness and enhancement of the membrane conductivity, with an apparent optimum metal concentration for the water-splitting reaction.
Similar content being viewed by others
References
Bauer, B., Gerner, F. J. and Strathmann, H., “Development of Bipolar Membranes,”Desalination,68, 279 (1988).
Cherif, A. T., Molenat, J. and Elmidaoui, A., “Nitric Acid and Sodium Hydroxide Generation by Electrodialysis using Bipolar Membranes,”J. Appl. Electrochem.,27,1069 (1997).
Chiao, Y. C., Chlanda, F. P. and Mani, K. N., “Bipolar Membranes for Purification of Acids and Bases,”J. Memb. Sci.,61, 239 (1991).
Choi, J.-S., Song, I.-K. and Lee, W.-Y., “A Composite Catalytic Polymer Membrane Reactor Using Heteropolyacid-Blended Polymer Membrane,”Korean J. Chem. Eng.,17, 280 (2000).
Graillon, S., Persin, F., Pourcelly, G. and Gavach, C., “Development of Electrodialysis with Bipolar Membrane for the Treatment of Concentrated Nitrate Effluents,”Desalination,107, 159 (1996).
Hanada, P. et al, US Patent 5,221,455 (1993).
Jialin, L., Yazhen, W., Changying, Y., Guangdou, L. and Hong, S., “Membrane Catalytic Deprotonation Effects,”J. Memb. Sci.,147, 247 (1998).
Kim, H.-S, Han, J.-W., Chun, K.-Y., Shul, Y.-G. and Joe, Y.-I., “Synthesis of Organic-Inorganic Composite Membrane by Sol-Gel Process,”Korean J. Chem. Eng.,12, 405 (1995).
Kim, Y.-H. and Moon, S.-H., “Lactic Acid Recovery from Fermentation Broth Using One-stage Electrodialysis,”J. Chem. Technol. and Biotechnol.,176, 1 (2001).
Lee, E.-G., Moon, S.-H., Chang, Y.-K., Yoo, I.-K. and Chang, H.-N., “Lactic Acid Recovery using Two-stage Electrodialysis and its Modeling,”J. Memb. Sci.,145, 53 (1998).
Lee, J.-K., Song, I.-K. and Lee, W.-Y., “Separation of H2/CO by The Selective Sorption Property of H3Pmo12O40 Embedded in Polyvinylalchol Membrane,”Korean J. Chem. Eng.,12, 384 (1995).
Liu, K. J., Chlanda, F. P. and Nagasubramanian, K., “Application of Bipolar Membrane Technology: A Novel Process for Control of Sulfur Dioxide from Flue Gases,”J. Memb. Sci.,3, 57 (1978a).
Liu, K. J., Nagasubramanian, K. and Chlanda, F. P., “Membrane Electrodialysis Process for Recovery of Sulfur Dioxide from Power Plant Stack Gases,”J. Memb. Sci.,3, 71 (1978b).
Mafe, S. and Manzanares, J. A., “Model for Ion Transport in Bipolar Membranes,”Physical Review A,42, 6245 (1990).
Mani, K. N., Chlanda, F. P. and Byszewski, C. H., “Aquatech Membrane Technology for Recovery of Acid/Base Values from Salt Streams,”Desalination,68,149 (1988).
Nagasubramanian, K., Chlanda, F. P. and Liu, K. J., “Use of Bipolar Membranes for Generation of Acid and Base — An Engineering and Economic Analysis,”J. Memb. Sci.,2, 109 (1977).
Onsager, L., “Deviation from Ohms Law in Weak Electrolytes,”J. Chem. Phys.,2, 599 (1934).
Pineri, M., Jesior, J. C. and Coey, J. M. D., “Iron Oxide Particles in a Perfluorosulfonate Membrane,”J. Memb. Sci.,24, 325 (1985).
Pomogailo, A. D., “Polymeric Immobilized Metal Complex Catalysts,” Moscow: Nauka (1988).
Ramirez, P., Aguilella, V. M., Manzanares, J. A. and Mafe, S., “Effects of Temperature and Ion Transport on Water Splitting in Bipolar Membranes,”J. Memb. Sci.,73,191 (1992).
Salamone, J. C., “Polymeric Materials Encyclopedia,” CRC press (1996).
Sata, T., “Properties of Ion-Exchange Membranes Combined Anisotropically with Conducting Polymers. 2. Relationship of Electrical Potential Generation to Preparation Conditions of Composite Membranes,”Chem. Mater.,3, 838 (1994).
Shimizu, K. and Tanioka, A., “Effect of Interface Structure and Amino Groups on Water Splitting and Rectification Effects In Bipolar Membranes,”Polymer,38,5441 (1997).
Simons, R., “Novel Method for Preparing Bipolar Membranes,”ElectrochimicaActa,31,1175 (1986).
Simons, R., “Preparation of a High Performance Bipolar Membrane,”J. Memb. Sci.,78,13 (1993).
Simons, R., “Water Splitting in Ion Exchange Membranes,”Electrochimica Acta,30, 275 (1985).
Strathmann, H., Krol, J. J., Rapp, H. J. and Eigenberger, G., “Limiting Current Density and Water Dissociation in Bipolar Membranes,”J. Memb. Sci.,125, 123 (1997).
Strathmann, H., Rapp, H. J., Bauer, B. and Bell, C. M., “Better Bipolar Membranes,”Chemtech, 17 (June 1993b).
Strathmann, H., Rapp, H. J., Bauer, B. and Bell, C. M., “Theoretical and Practical Aspects of Preparing Bipolar Membranes,”Desalination,90, 303 (1993a).
Tanioka, A., Shimizu, K., Miyasaka, K., Zimmer, H. J. and Minoura, N., “Effect of Polymer Materials on Membrane Potential, Rectification and Water Splitting in Bipolar Membranes,”Polymer,37,1883 (1996).
The Membrane Society of Korea, “Membrane Separation,” Freedom Academy, Korea (1996).
Tokuyama Co. Ltd., Bulletin-NEOSEPTA Ion Exchange Membrane (1993).
Zabolotskii, V. I., Ganych, V. V. and Sheldeshov, N. V., “Effect of Copper Complexes with the Ionogenic Groups of the MA-40 Anion-Exchange Membrane on the Dissociation Rate of Water Molecules in the Electric Field,”Sov. Electrochem.,27, 1098 (1991).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, MS., Tanioka, A. & Moon, SH. Effects of interface hydrophilicity and metallic compounds on water-splitting efficiency in bipolar membranes. Korean J. Chem. Eng. 19, 99–106 (2002). https://doi.org/10.1007/BF02706881
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706881